Presentation and Initial Work-up
A 38-year-old Caucasian female presented with a one-day history of decreased vision and burning sensation in her right eye. Two days prior to presentation, she was planting flowers in her garden to attract more butterflies to her yard. She reported mild irritation in her right eye immediately after completing the yard work, which improved with lubrication. The next morning, she woke up with blurry vision followed by progressive foreign body sensation in her right eye. She also reported photophobia but denied headache, history of trauma, recent illness, or concurrent symptoms in the left eye. Systemic review was negative; the patient denied history of any cold sores, rashes, joint pain, new medications or the use of any eye drops.
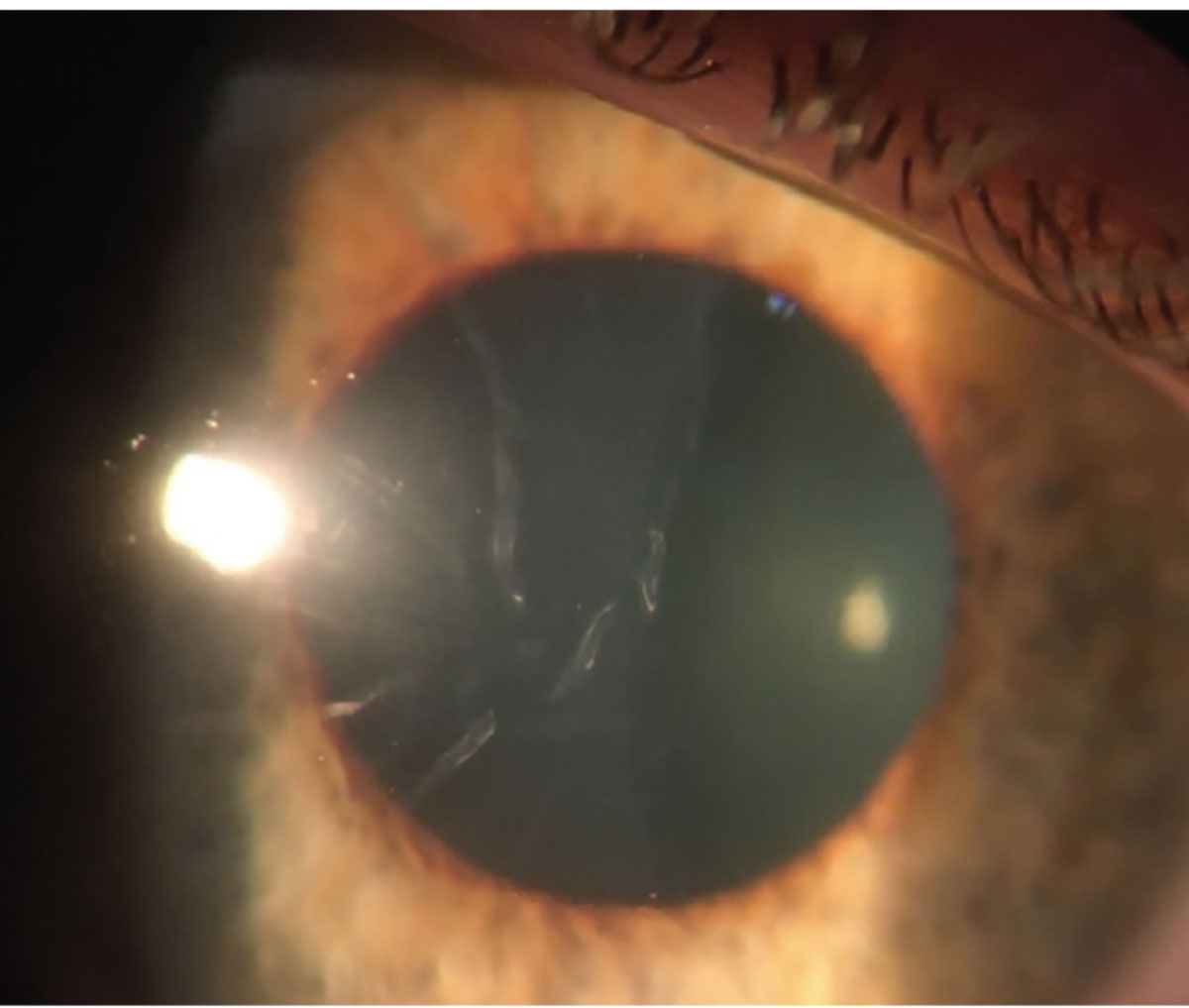 |
| Figure 1. Slit lamp photo of the right eye on the day of presentation showing moderate Descemet’s folds but an otherwise clear cornea. |
Medical History
Our patient had a right dacryocystorhinostomy 10 years prior but denied any other past ocular history. Past medical history was significant only for GERD and mild iron-deficiency anemia, for which the patient takes omeprazole and an over-the-counter iron supplement daily. Family history and social history were also unremarkable.
Exam
Initial ocular examination demonstrated a best corrected visual acuity of 20/70 OD and 20/20 OS. External examination was normal with no rashes, edema or erythema. Extraocular motility, confrontational visual field testing, and pupillary exam were normal. Intraocular pressures were 14 mmHg bilaterally. Lid eversion of the right eye revealed mild conjunctival injection and a papillary reaction without any foreign body. The pH of the ocular surface was normal.
The patient’s right cornea was uniformly edematous, with stromal haze and 2+ Descemet folds throughout (See Figure 1). There was a small amount of scattered punctate epithelial erosions. The endothelium appeared otherwise normal, without any clear disruption of Descemet’s membrane detectable by slit lamp. The left cornea was normal. No stromal infiltrate or keratic precipitates were observed in either eye. Her anterior chambers were deep and quiet in both eyes, and the remainder of the examination, including a dilated fundoscopic exam, was normal.
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
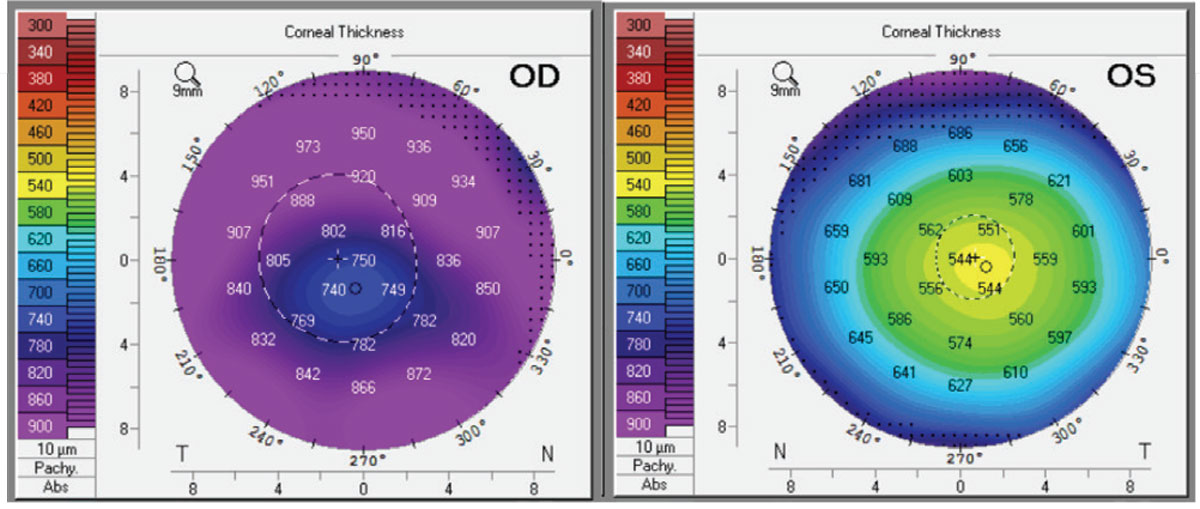 |
| Figure 2. Corneal thickness by Pentacam of right and left eyes on day of presentation. Click image to enlarge. |
Ancillary imaging was obtained, including Pentacam (See Figure 2), specular microscopy (See Figure 3), and anterior segment OCT (See Figure 4). Pentacam revealed severe unilateral corneal edema. Endothelial cells were undetectable in the right eye by specular microscopy, while the left eye had an endothelial cell density of 2,439 cells/mm2.
The differential diagnosis for this patient with sudden onset of unilateral corneal edema includes endothelial dystrophy, infection, inflammation and metabolic etiologies. The presentation was not consistent with an endothelial dystrophy as the patient’s age and
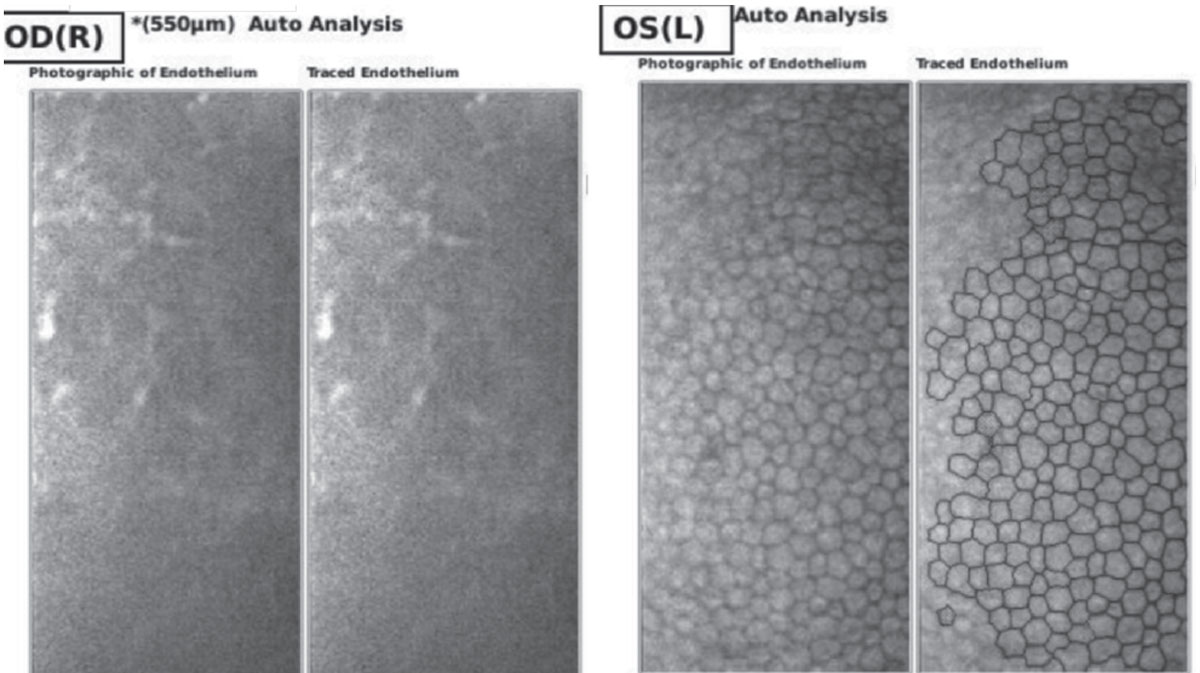 |
| Figure 3. Endothelial cell density by specular microscopy of right and left eyes on day of presentation, demonstrating inadequate visualization of endothelial cells of the right eye and normal endothelium of the left eye. |
sudden onset are atypical for a first presentation of endothelial dystrophy, her endothelium and stroma were clinically normal in both eyes, and the patient reliably denied any previous ophthalmic history.1,2 Infectious and inflammatory causes such as a herpes simplex viral keratitis/endotheliitis or anterior uveitis were also considered, but the patient’s complete lack of stromal, endothelial, or anterior chamber inflammation reduced the likelihood of these diagnoses. Upon a more detailed review of the patient’s history, she revealed she was planting milkweed flowers and handling them without gloves, leading to a presumed diagnosis of corneal edema secondary to acute endothelial toxicity.
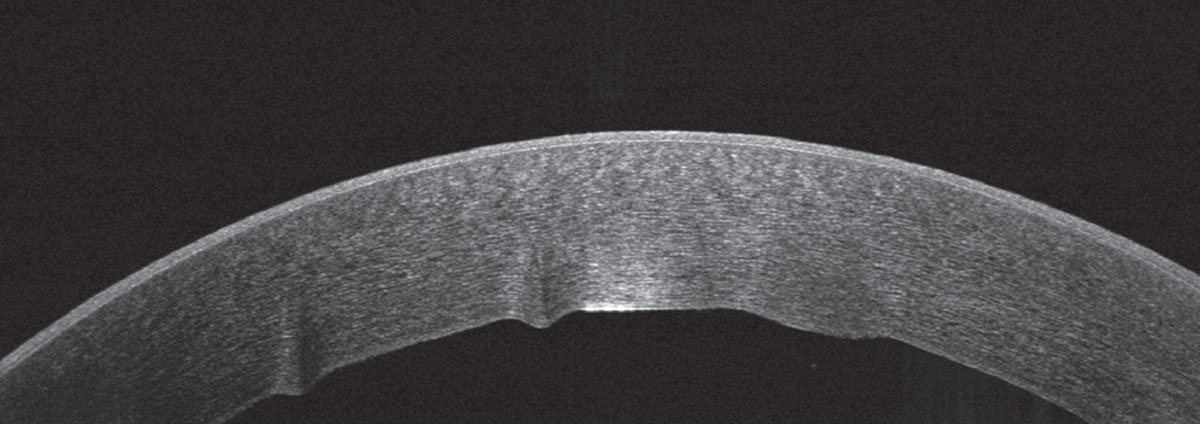 |
| Figure 4. Anterior segment OCT of the right eye on the day of presentation, demonstrating stromal edema and Descemet folds. |
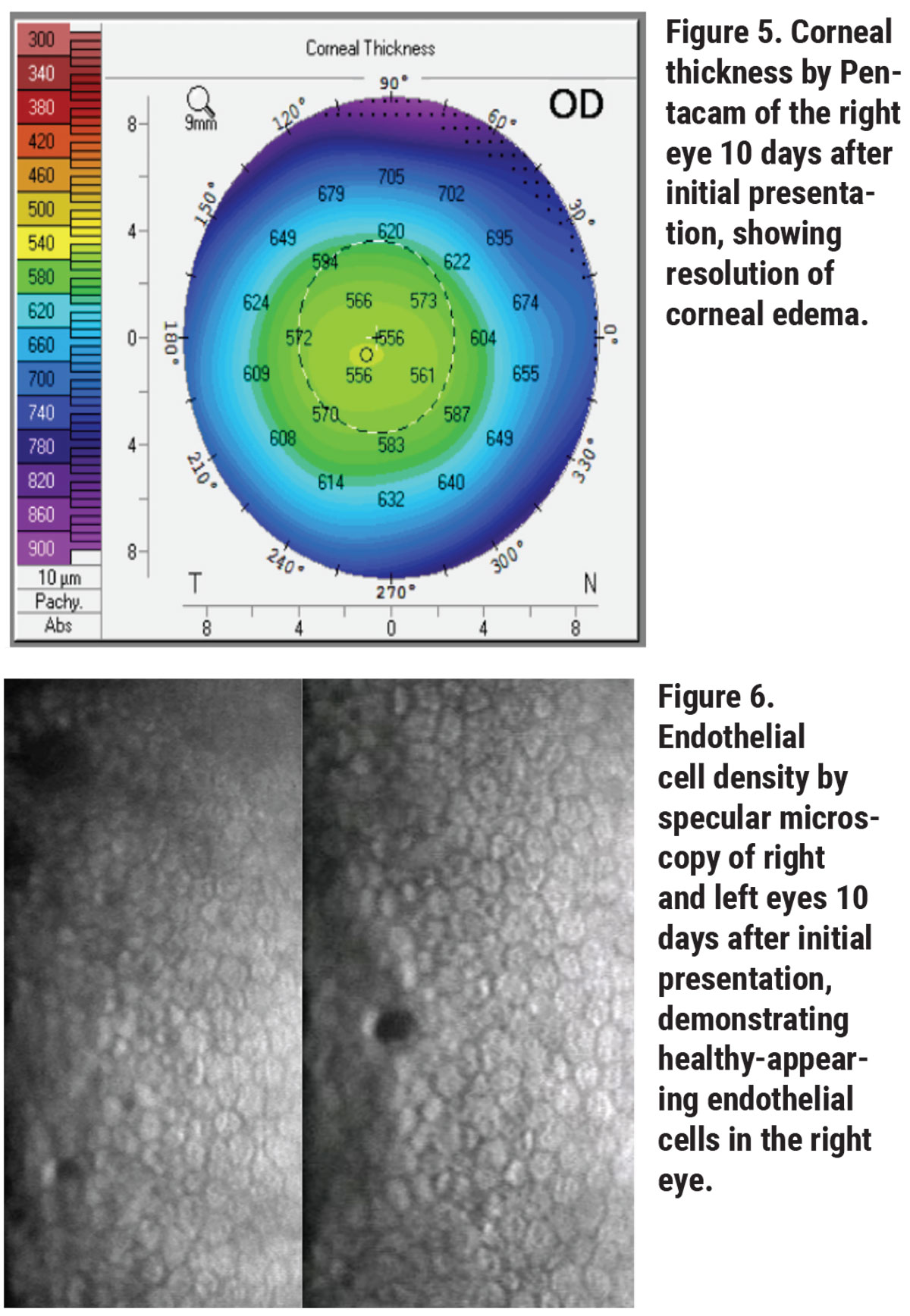
Her corneal edema in the right eye was empirically treated with loteprednol 0.5% every two hours while awake and with Muro 128 5% saline drops four times per day. The patient was also counseled on avoiding further milkweed exposure. She returned to clinic the next day and her vision and corneal edema had improved, but she complained of worsening foreign body sensation. Slit lamp examination revealed a new, 3-mm, ruptured central bulla without infiltrate. A bandage contact lens was placed and the patient continued the same drop regimen with close follow-up. Two days later, her epithelial defect had closed and her corneal edema had further improved. Her bandage contact lens was then removed and her drops tapered, and within 10 days of initial presentation, her right eye’s visual acuity returned to its baseline of 20/20. Repeat Pentacam, specular microscopy and anterior segment OCT showed rapid recovery, with endothelial cell density recovering to 2,349 cells/mm2 in the right eye to match the left eye cell density of 2,439 cells/mm2 (See Figures 5, 6, 7).
Discussion
 |
| Click image to enlarge. |
Milkweed plants, formally known as plants belonging to the genus Asclepias, are relatively common wildflowers known for their ability to attract butterflies and other insects. In particular, the Monarch butterfly is drawn to the plant, making it an increasingly popular flower in many gardens.3 Milkweed sap contains high levels of cardenolides, also known as cardiac glycosides, which inhibit the cellular sodium-potassium adenosine triphosphatase (Na+/K+-ATPase) pump.3,4 Digoxin, the cardiac inotropic medication, is an example of a low-dose cardiac glycoside used to increase heart function. However, cardiac glycosides are very potent cytotoxins and have been known to kill large animals when grazing on a single milkweed plant.3,5 Interestingly, Monarch butterflies are unique among animals and have mutated Na+/K+-ATPase pumps that render them immune to cardenolides toxicity.6,7
In the eye, the endothelial cells that maintain corneal transparency are highly metabolically active and rely primarily on the Na+/K+-ATPase pump to dehydrate the cornea.8,9 Both topical and systemic digoxin exposure cause dysfunction of endothelial cells, leading to rapid-onset corneal edema and bullous keratopathy.9 In almost all cases, endothelial function returns within several days after cessation of cardenolide toxin exposure if the ensuing edema and inflammation can be controlled in the interim.10,11
Steroid drops play an important role in recovering from toxic corneal edema. Especially in younger patients, steroids protect endothelial cells during recovery by reducing cellular edema and inflammation.9,13 Steroids have also been demonstrated to upregulate Na+/K+-ATPase pump activity, which could increase toxin clearance by the remaining functional endothelial cells.12 Some cases even report using oral prednisone in addition to topical steroids to hasten endothelial recovery.10,11 Muro 128 drops may also accelerate visual improvement by controlling corneal edema while the endothelium recovers.9,13
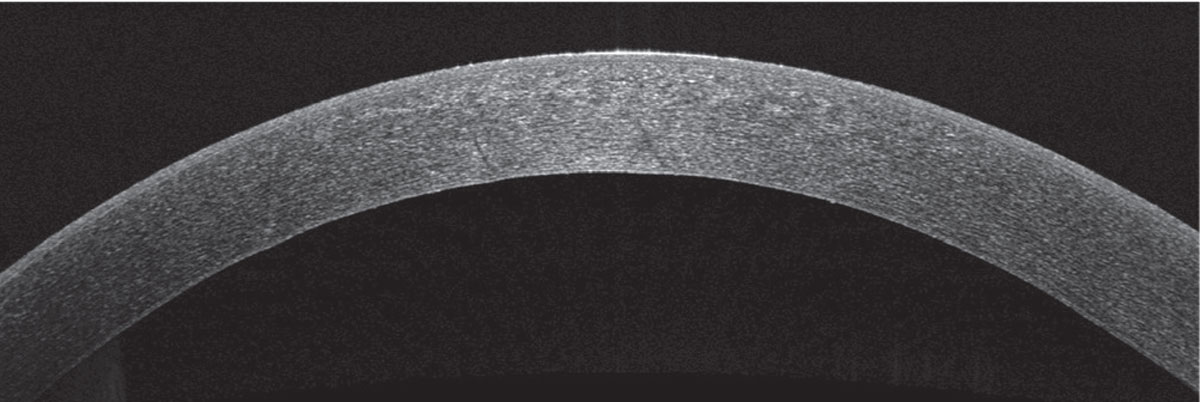 |
| Figure 7. Anterior segment OCT of the right eye 10 days after first presentation, showing resolution of stromal edema and Descemet’s folds. |
In summary, endothelial milkweed toxicity is increasingly common and difficult to diagnose, especially if the patient does not remember milkweed contact as even small amounts of cardenolide toxin exposure can cause significant endothelial dysfunction. The presentation can also be confused for decompensated Fuchs dystrophy or HSV endothelial keratitis, especially if advanced corneal imaging techniques are not available. Misdiagnosis may delay the use of steroids, which could worsen endothelial inflammation and cause permanent endothelial loss.9 Fortunately, if long-standing endothelial edema and inflammation are avoided, patients generally make a full recovery within several days without long-term complications.
1. Aldave AJ, Han J, Frausto RF. Genetics of the corneal endothelial dystrophies: An evidence-based review. Clin Genet 2013;84:109-19.
2. Gottsch JD, Sundin OH, Liu SH, Jun AS, Broman KW, et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci 2005;46:1934–39.
3. Joubert, J. Toxicants of plant origin. Toxicants of Plant Origin. CRC Press, 1989. 61-96.
4. Madreperla SA, Johnson M, O’Brien T. Corneal endothelial dysfunction in digoxin toxicity. Am J Ophthalmol 1992;113:2:211-2.
5. Benson JM, Seiber JN, Bagley CV, Keeler RF, Johnson AE, Young S. Effects on sheep of the milkweeds Asclepias eriocarpa and A. labriformis and of cardiac glycoside-containing derivative material. Toxicon 1979;17:2:155-65.
6. Elsner E. Monarchs and milkweed: A migrating butterfly, a poisonous plant, and their remarkable story of coevolution by Anurag Agrawal (book review). American Entomologist 2017;63:3:197.
7. Pocius VM, Debinski DM, Pleasants JM, Bidne KG, Hellmich RL, Brower LP. Milkweed matters: Monarch butterfly (Lepidoptera: Nymphalidae) survival and development on nine Midwestern milkweed species. Environ Entomol 2017;46:5:1098-1105.
8. Madreperla SA, Johnson M, O’Brien T. Corneal endothelial dysfunction in digoxin toxicity. Am J Ophthalmol 1992;113:2:211-2.
9. Lee YJ, Han SB, Hyon JY. Corneal endothelial dysfunction caused by Asclepias curassavica in a young farmer. Am J Ophthalmol Case Rep 2019;16:100564.
10. Duncker G, Krastel H. Ocular digitalis effects in normal subjects. Lens Eye Toxic Res 1990;7:3:281-303.
11. Smith JL, Mickatavage RC. The ocular effects of topical digitalis. Am J Ophthalmolo 1963; 56:6:889-894.
12. Hatou S, Yamada M, Mochizuki H, et al. The effects of dexamethasone on the Na, K-ATPase activity and pump function of corneal endothelial cells. Curr Eye Res 2009;34:5:347-354.
13. Amiran MD, Lang Y, Yeung SN. Corneal endothelial toxicity secondary to Asclepias fruticosa. Eye (Lond) 2011;25:7:961-963.