Workup, Diagnosis and Treatment
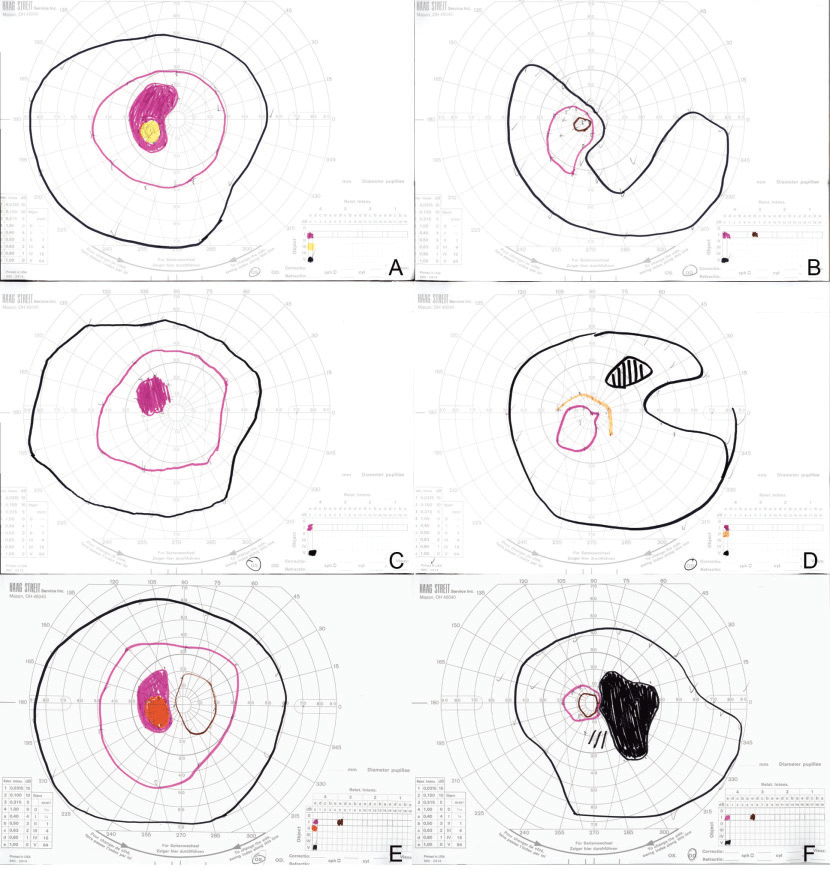
OCT demonstrated mild thinning of the macula in both eyes, with possible abnormalities of the ellipsoid zone in the right eye (Figure 2 A,B). Fluorescein angiography was within normal limits. Fundus autofluorescence demonstrated a mild salt-and-pepper appearance in the right eye (Figure 1 C,D). Humphrey perimetry of her right eye demonstrated superotemporal constriction, almost in the form of a superotemporal arcuate scotoma, denser temporally, as well as an inferior arcuate scotoma. This was reproduced with Goldmann perimetry (Figure 3 A,B).
At this point, the differential diagnoses included the spectrum of acute zonal occult outer retinopathies, autoimmune retinopathy, and, less likely, cancer-associated retinopathy. Given the patient’s severe subjective visual field worsening over a period of days, she was admitted for intravenous methylprednisolone 250 mg every six hours and further work-up. Full-field ERG demonstrated cone dysfunction in the right eye, but was normal in the left eye. Visual evoked potential demonstrated a delay in the right eye but was normal in the left. An MRI of the brain and orbits, with and without gadolinium, was unremarkable. A computed tomography scan of the chest, abdomen and pelvis showed a benign right lung nodule and small benign-appearing hepatic cysts, but no evidence of malignancy. C-reactive protein, erythrocyte sedimentation rate and complete blood count were normal. An autoimmune retinopathy panel was sent to the Casey Eye Institute and was pending at the time of discharge. She was discharged after 12 doses of IV steroids with an oral prednisone taper (60 – 40 – 30 – 20 – 10 mg daily, tapered every three days).
Two weeks after discharge, the patient’s symptoms and Goldmann visual field improved dramatically (Figure 3 C,D). Four weeks after initial presentation, the autoimmune retinopathy panel arrived, showing positivity for several anti-retinal auto-antibodies, including HSP27, aldose, enolase, arrestin and glyceraldehyde 3-phosphate dehydrogenase. Recoverin, PKM2, tubulin and carbonic anhydrase II autoantibodies were absent. Based on the above findings, the patient’s presentation was consistent with non-paraneoplastic autoimmune retinopathy.
She received intravitreal triamcinolone in the right eye, but denied any improvement with it. Her Goldmann visual field 10 weeks after initial presentation re-demonstrated a superotemporal scotoma in her right eye with progression toward her central vision (Figure 3 E,F). Intravenous steroid therapy was reinitiated and followed with a slow oral steroid taper (methylprednisolone 1 g daily for three doses, then 60 – 40 – 20 – 10 mg daily, tapered every two weeks; then 10 mg every other day). This was administered in conjunction with starting mycophenolate mofetil 1,000 mg b.i.d. Assessment of treatment response is ongoing.
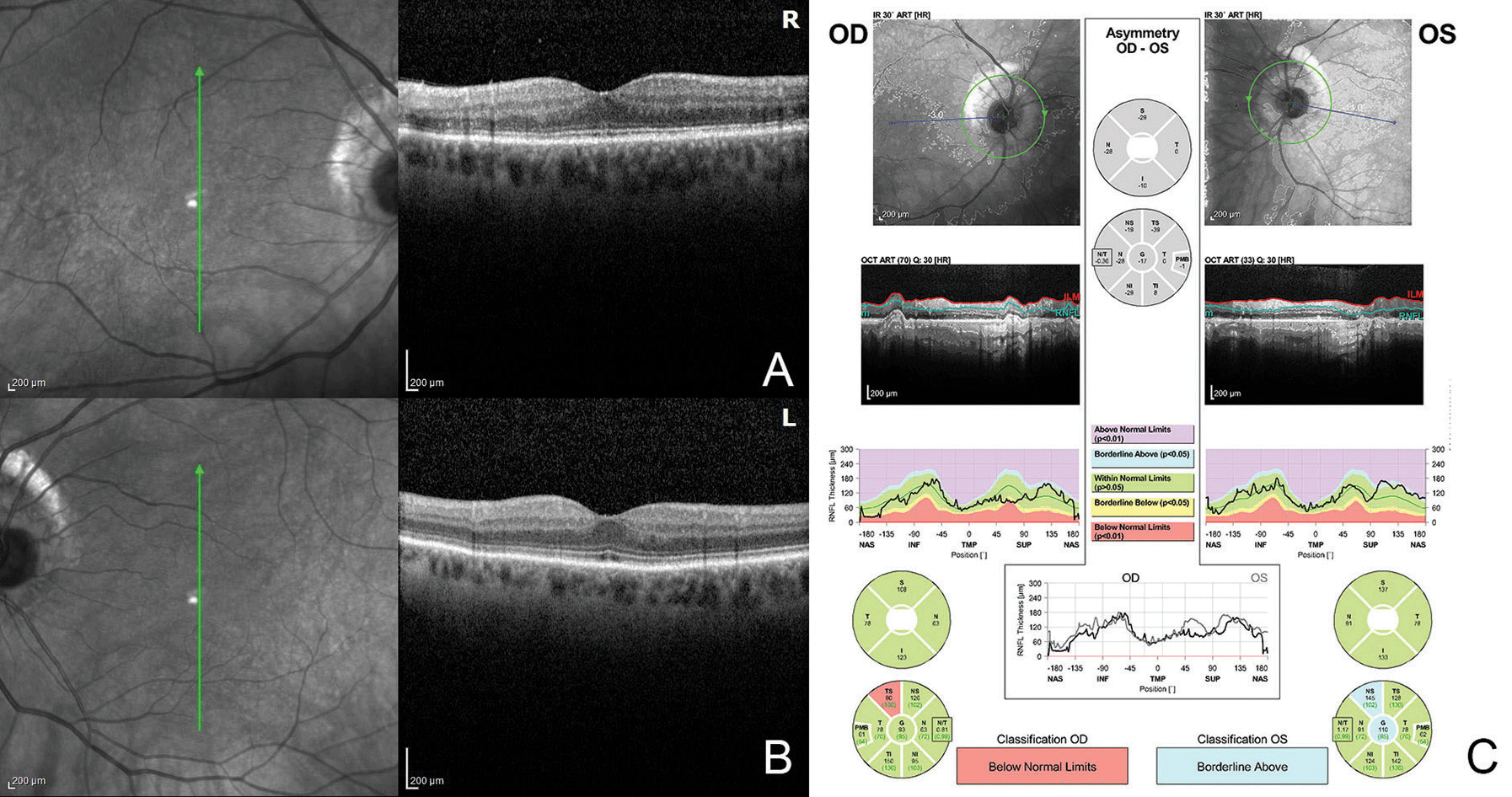 |
Figure 2. (A,B) OCT demonstrates mild thinning of the macula in both eyes with possible abnormalities of the ellipsoid zone in the right eye. OCT of the optic nerves (C) shows superotemporal thinning in the right eye but is otherwise normal. |
 |
|
|
Discussion
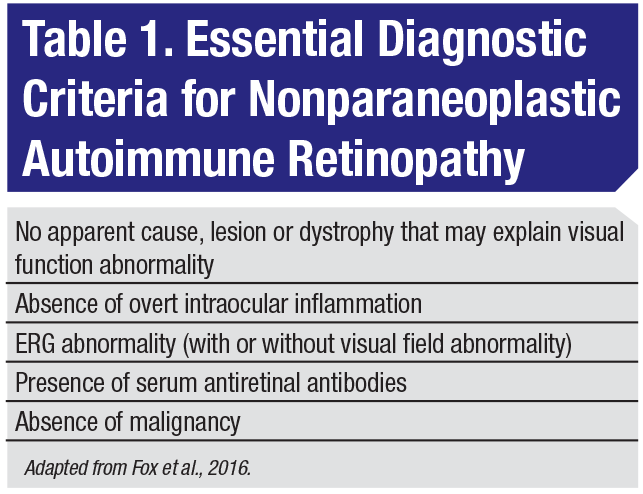
Autoimmune retinopathy describes a diverse group of conditions with antiretinal autoantibodies implicated in the etiology. AIR is subdivided into paraneoplastic and nonparaneoplastic subtypes, depending on the presence or absence of concomitant malignancy. While universally-accepted diagnostic criteria for nonparaneoplastic AIR remain elusive, a recent expert consensus-driven effort developed several essential diagnostic criteria (Table 1).1 These include: visual function abnormality without apparent cause or malignancy; absence of inflammation; ERG abnormality with or without a visual field abnormality; and the presence of serum antiretinal antibodies.
The differential diagnosis of nonparaneoplastic AIR includes drug toxicity, vitamin deficiencies, occult posterior uveitis, acute zonal occult outer retinopathy, inherited retinal degenerations and carcinoma- or melanoma-associated retinopathy. While drug toxicity and vitamin deficiency may be excluded with history, additional testing is often employed to investigate the cause. This testing includes OCT, fluorescein angiography, fundus autofluorescence, genetic testing and malignancy evaluation in conjunction with other specialists.1,2
Testing for serum antiretinal antibodies remains non-standardized and commercially available on a limited basis.1 Consequently, concordance between laboratories may be low. One study found only 64-percent concordance between two laboratories for finding the presence of any antiretinal antibodies and 36 percent for having a positive result for a specific antiretinal antibody. The potential for such disparate results may lead clinicians to send serum samples to at least two labs.3
Evidence supporting the treatment of AIR is based largely on observational studies.4 One retrospective series of 30 patients treated with systemic or local immunosuppression found that 70 percent demonstrated improvement in visual acuity or a visual field deficit.5 More recently, a majority of patients with autoimmune retinopathy treated with rituximab demonstrated stable or improved visual acuity six months after therapy initiation.6
 |
Our 72 year-old patient met the essential diagnostic criteria developed by expert consensus.1 While her clinical exam was unrevealing, ancillary studies were notable for cone dysfunction on ERG, visual field deficit on Humphrey and Goldmann perimetry, and ellipsoid zone abnormalities on OCT. The presence of serum antibodies was confirmed; however, due to a testing turnaround time of two to four weeks and evidence that a delay in treatment may portend a worse prognosis,6 treatment with systemic steroids was initiated prior to antiretinal autoantibody confirmation. While her visual field defect improved with steroids, she relapsed while off them and is now currently being treated with steroid-sparing immunosuppression. REVIEW
1. Fox AR, Gordon LK, Heckenlively JR, et al. Consensus on the diagnosis and management of nonparaneoplastic autoimmune retinopathy using a modified Delphi approach. Am J Ophthalmol 2016;168:183–190.
2. Sorbin L. Progress toward precisely diagnosing autoimmune retinopathy. Am J Ophthalmology 2018;188:xiv-xv.
3. Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol 2013;131:113–115.
4. Fox AR, Sen HN, Nussenblatt RB. Autoimmune retinopathies. In: Ryan’s Retina, 6th ed. London: Elsevier, 2017:1562-1571.
5. Ferreyra HA, Jayasundera T, Khan NW, et al. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol 2009;127:390–397.
6. Davoudi S, Ebrahimiadib N, Yasa C, et al. Outcomes in autoimmune retinopathy patients treated with rituximab. Am J Ophthalmol 2017;180:124–13.



