Rhegmatogenous retinal detachment can lead to profound loss of vision. Surgical techniques for repairing RRD have advanced considerably since the days of Jules Gonin in the early 20th century, however.1 While scleral buckling has historically been the gold standard form of treatment of RRD, primary pneumatic retinopexy and pars plana vitrectomy surgery have been employed with increasing frequency. We review here the fundamental principles of RRD as well as the literature regarding the role of primary scleral buckling, PR and PPV in RRD repair.
Fundamentals: Retinal Tears and Detachment
Retinal detachment is defined as a separation of the neurosensory retina from the retinal pigment epithelium, at the level of the photoreceptor outer segments. There are two types of retinal detachments: rhegmatogenous and non-rhegmatogenous. The distinguishing characteristic is the presence or absence of a full-thickness retinal break in patients with RRD, which is always present, even if it cannot be located by ophthalmoscopy. Breaks, or "rhegma," are subdivided into tears, holes and dialyses. RRD typically results in a corrugated appearance to the retina secondary to the presence of subretinal fluid (See Figure 1). 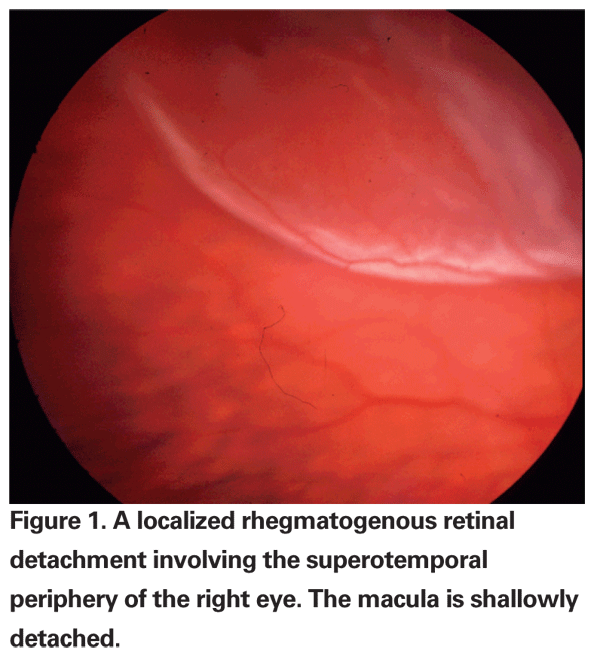
"Horseshoe tears" are the most common breaks causing RRDs and result from antero-posterior vitreous traction along the posterior margin of the vitreous base (See Figure 2). Retinal tears may occur along the edges of lattice degeneration, chorioretinal scars, or in areas of apparently normal retina. Typically, an RRD is caused by a retinal tear occurring in the setting of a posterior vitreous separation. Retinal tears that involve in excess of three clock hours are referred to as giant retinal tears (See Figure 3) and are associated with a high risk of proliferative vitreoretinopathy.
Retinal holes may be operculated or atrophic. An operculated hole occurs when vitreous traction amputates the flap of the tear from the retinal surface and the separated flap becomes suspended within the posterior hyaloid above the retinal surface. In contrast, an atrophic, or round, hole is not caused by traction. Both operculated and atrophic holes are usually found at the posterior margin of the vitreous base. Atrophic holes are often found within or adjacent to areas of lattice degeneration. Neither type of hole is likely to cause an RRD.
A retinal dialysis is a circumferential break occurring at the ora serrata, as opposed to the more typical posterior margin of the vitreous base. Dialyses typically occur following blunt trauma.
Pathogenesis of RRD
The pathogenesis of RRD is well-understood.2 Around any retinal break exists a balance of forces. Some forces maintain RPE and retina apposition, while other forces promote retinal detachment. Most retinal breaks do not cause or lead to an RRD. Rather, detachments occur when the forces promoting retinal detachment overwhelm the forces maintaining retinal attachment.
Forces maintaining retinal attachment include hydrostatic pressure, oncotic pressure and active transport. Intraocular pressure creates a higher hydrostatic pressure within the vitreous than within the choroid. The choroid also contains more dissolved substances than the vitreous and thus has a higher oncotic pressure. Finally, the RPE pump actively transports solutes and fluid from the subretinal space into the choroid. These three forces result in a net vitreous-to-choroid fluid vector that tends to maintain retinal apposition.
Forces promoting retinal detachment include vitreous traction, gravity and eye movements. Active traction on the flap of a horseshoe tear accelerates the passage of liquid vitreous into the subretinal space. Gravity promotes the spread of subretinal fluid, particularly with superior breaks. Eye movements are probably the least powerful of these three mechanisms, but do appear to accelerate RRD formation, at least in some cases.
Methods of Surgical Repair
The two most important factors in the development of RRD are retinal breaks and vitreous traction. Therefore, the two goals of retinal reattachment surgery are to seal all retinal breaks and to relieve all vitreous traction. Most early surgical failures are due to the inability to achieve one or both of these goals. Late surgical failures are typically due to development of PVR (See Figure 4).
The most common surgical options for management of retinal detachments are scleral buckling, PR, and/or PPV. Other less-common options include observation, laser barricade and the Lincoff-Kreissig parabulbar balloon (which is currently unavailable in the
Scleral Buckling
Scleral buckling most likely works through a variety of mechanisms, but the most important is believed to be the indirect relief of radial vitreous traction.3 In addition, scleral buckling probably displaces some subretinal fluid away from the break.
Surgeons may perform an encircling scleral buckle, a segmental buckling element or a combination of the two methods. An encircling buckle has more extensive support and will even support an untreated retinal break (See Figure 5). A segmental buckle will support localized breaks and also has a lower complication rate. The materials used are made of either solid silicone rubber or silicone sponges. Drainage of subretinal fluid can be performed with either type of buckling procedure, but is typically performed for large bullous retinal detachments. Most cases can probably be managed without drainage, however.
Single operation success rates for scleral buckling are often reported to be greater than 90 percent (See Figure 6). In addition, scleral buckling has certain advantages over other techniques. It is the most established procedure with the longest documented follow-up4 and is appropriate for almost all primary RRDs. Exceptions include giant retinal tears, posterior breaks, multiple breaks in the setting of necrotizing herpetic retinitis and patients in whom scleral buckling is technically too difficult (i.e., thin sclera, history of either a glaucoma drainage device or multiple strabismus surgeries). An intraocular tamponade agent, such as gas or silicone oil, is oftentimes not required, and positioning is typically supine, which is tolerated well by most patients. 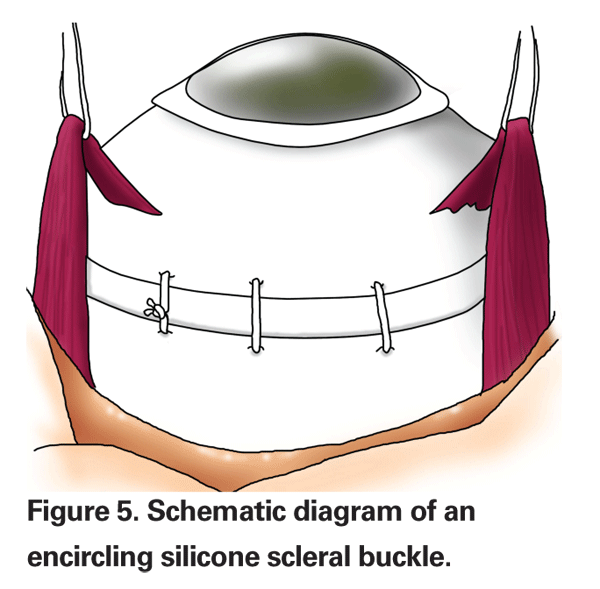
Complications from scleral buckling surgery include axial myopia, seen with encircling elements and strabismus. Less-common complications include infected buckling elements, buckle extrusions through the conjunctiva, or intrusions through the sclera, and anterior segment ischemia. Complications from drainage procedures include hemorrhage and either vitreous or retinal incarceration.
Pars Plana Vitrectomy
PPV surgery allows for direct relief of vitreous traction associated with retinal breaks. It can also be used to treat a variety of situations typically not amenable to repair by scleral buckling such as posterior retinal breaks, retinal detachments associated with vitreous hemorrhage or that occur in the setting of necrotizing herpetic retinitis, giant retinal tears and detachments complicated by the formation of PVR. PPV also allows for controlled drainage of subretinal fluid either with heavy perfluorocarbon liquids or internal drainage techniques. This allows for complete intraoperative retinal reattachment. At the conclusion of surgery, air, gas or silicone oil must be injected into the vitreous cavity to tamponade the retinal break(s) until the retinopexy (either with cryopexy or laser) provides a permanent seal. Air or gases have the advantage of spontaneously resorbing during the postoperative period. In contrast, silicone oil requires a second PPV procedure for removal. Patients generally tolerate the surgery well with regard to pain level compared with scleral buckling surgery. The single operation success rates for PPV have been reported to be in the range of 85 to 90 percent.5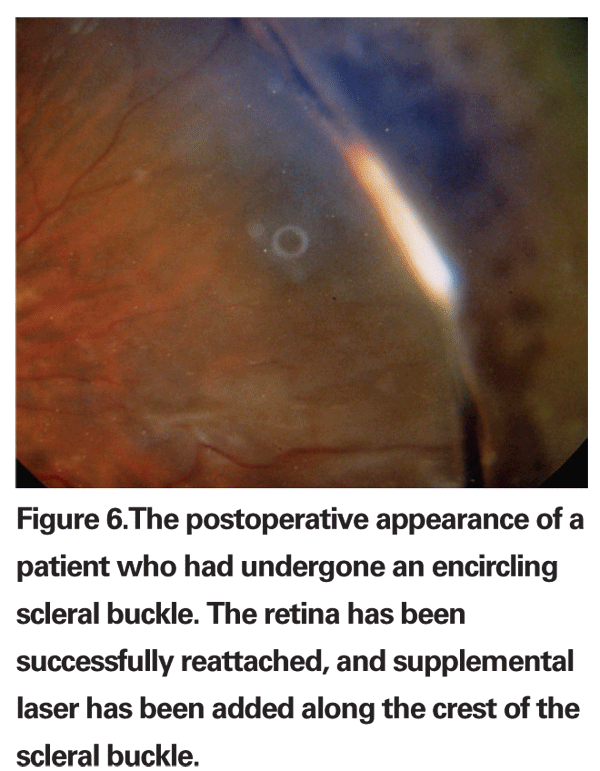
Complications from PPV include iatrogenic cataract, elevated intraocular pressure (especially from expansile gases), formation of new intraoperative retinal breaks and retinal or optic nerve trauma from instrumentation. Postoperative positioning is oftentimes required for a short period of time, dependent upon the location of the retina pathology.
Pneumatic Retinopexy
Pneumatic retinopexy typically utilizes an intraocular gas bubble to provide temporary tamponade of the retinal break(s) until the retinopexy, either with cryopexy or laser, provides a permanent seal (See Figure 7).6 It does not relieve vitreous traction, however. The retina becomes reattached mainly through the actions of the RPE pump. Typically, less than 1 cc of gas (either C3F 8 or SF6) is utilized, and it will have an arc of contact of approximately one quadrant (three clock hours), so precise head positioning is required after the procedure.
The indications for PR are relatively limited, though they vary from surgeon to surgeon. The ideal candidate for PR is a phakic patient with an RRD with one break, or several closely spaced breaks in the superior clock hours. The morbidity from the procedure is very low as it an office procedure with no conjunctival incisions.
Single operation success rates are lower than those of scleral buckling or PPV. The reported anatomic success rates are in the range of 75 to 80 percent.7 Complications include new postoperative retinal breaks, intraocular pressure spikes from the expansile gas which may be employed, along with cataract formation.
Other Procedures and Treatments
Two other procedures, the Lincoff-Kreissig orbital balloon and laser barricade, are used infrequently. The Lincoff-Kreissig orbital balloon, which is currently not available in the 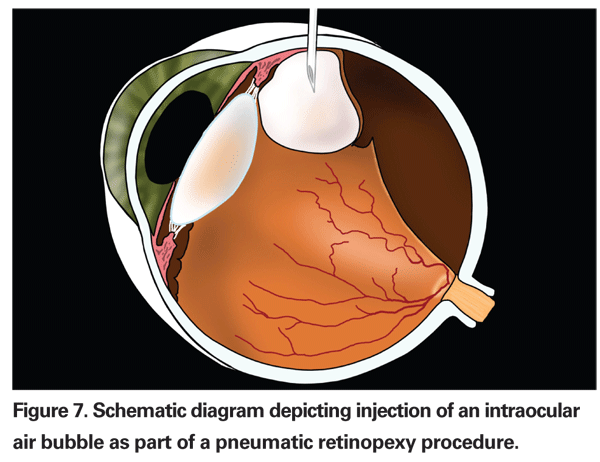
Laser or cryopexy used to wall off a limited, peripheral, macula-on RRD are also used infrequently and are not curative.9 This is most often done on patients for whom surgery is otherwise contraindicated. Long-term risks include PVR, hypotony, epiretinal membrane formation and rubeosis irides.
Observation of an asymptomatic RRD is appropriate in selected situations.10 Such is the case in younger patients with a localized detachment secondary to an atrophic hole, sometimes related to lattice degeneration, particularly when demarcation lines are present. This, of course, is not curative, and these patients are subject to long-term risks similar to those of eyes treated with laser demarcation.
Choice of Repair
The two most commonly utilized techniques for repair of an RRD are scleral buckling and PPV. There is still no consensus in the vitreoretinal community regarding the primary management of RRD. Historically, PPV was considered second-line treatment for primary RRDs, especially in phakic patients. However, with advances in wide-field viewing systems and new instrumentation, including 23- and 25-ga. vitrectomy systems, PPV has increased in popularity. Most vitreoretinal surgeons today prefer PPV in pseudophakic retinal detachments so as to not change the refractive status of the patient. It may also be easier to identify multiple, small peripheral retinal breaks, which are often seen in pseudophakic detachments.11
Traditional teaching held that PPV for primary repair of an RRD had too many disadvantages and/or complications, including a lower single-operation success rate. Multiple retrospective and prospective case series have now called this into question, however. In the vast majority of these case series, the single- operation success rate appears to be equivalent between scleral buckling and PPV, in both phakic and pseudophakic/ aphakic rhegmatogenous retinal detachments.12,13 Visual acuity outcomes also appear to be independent of macula-on/off status. While scleral buckling was initially reported in 1989 to decrease the risk of PVR,14 a report in 2005 found that scleral buckling may actually increase the risk of PVR.15
The Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment (SPR) study was a European multicenter, randomized, prospective, controlled clinical trial that compared the two treatment entities in both phakic and pseudophakic eyes.16,17 This study evaluated medium-complexity retinal detachments and had one-year follow-up. The results showed a benefit in scleral buckling over PPV in final best corrected visual acuity in phakic patients. In phakic patients, anatomic success rates and the rate of PVR were equal between the two groups. The investigators did see a higher anatomic success rate using PPV in patients with pseudophakic/aphakic retinal detachments, however. In addition, combined PPV and scleral buckling showed a trend toward a greater anatomic success rate in pseudophakic patients.
Author Recommendations
There are many different techniques to repair RRDs and the final choice on the type of procedure needs to be individualized for each patient and surgeon. Regardless of technique, the fundamental principles still need to be followed. The most critical step in the operation is the precise localization of all retinal breaks. A careful preoperative evaluation should include slit-lamp evaluation as well as binocular indirect ophthalmoscopy with scleral depression. This should be repeated in the operating room, as untreated retinal breaks usually lead to surgical failure.
For phakic patients with a superior detachment associated with a single break or multiple breaks in close proximity, we oftentimes employ PR. If the indications are not met for PR in a phakic patient, a segmental or encircling buckle is placed. In the setting of significant vitreous opacities, including vitreous hemorrhage, a PPV approach is preferred.
For pseudophakic detachments, we prefer a PPV without placement of a scleral buckle, as this is less likely to lead to a change in the refractive status of the patient. If the location of the retinal breaks cannot be determined, PPV is employed, as it is generally quite easy to find the site of the retinal tear(s) intraoperatively.
For giant retinal tears, we prefer a PPV approach, with scleral buckle support of the giant retinal tear. Inferior breaks are also repaired with a combination of scleral buckling with PPV. For dialyses, we perform an encircling buckle as well as PPV. For all cases which need intraocular tamponade, air or gas is preferred. Silicone oil is reserved for circumstances such as unusual positioning requirements (children, handicapped patients, air travel required), or the presence of significant PVR.
In summary, choosing the most appropriate type of surgery depends on a number of factors, such as the number, location and type of retinal breaks, lens status, presence of PVR and vitreous opacities. Regardless of the type of surgical modality chosen, the vast majority of RRDs can be successfully repaired with a single operation.
Dr. Lin is a vitreoretinal fellow and Dr. Mieler is a professor and chairman of the Department of Ophthalmology and Visual Science at the
1. Gonin J. The treatment of detached retina by searing the retinal tears. Arch Ophthalmol 1930;4: 621
2.
3. Thompson JT. The effects and action of scleral buckles in the treatment of retinal detachment. In: Ryan SJ, ed. Retina. 4e.
4. Schwartz SG, Kuhl DP, McPherson AR, et al. Twenty-year follow-up for scleral buckling. Arch Ophthalmol 2002;120:325-329.
5. Schwartz SG, Mieler WF. Management of primary rhegmatogenous retinal detachment. Comp Ophthalmol Update 2004;5:285-294.
6. Hilton GF, Grizzard WS. Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision. Ophthalmol 1986;93:626-641
7. Tornambe PE. Pneumatic retinopexy: The evolution of case selection and surgical technique—A twelve-year study of 302 eyes. Trans Am Ophthalmol Soc 1997;95:551-578.
8. Lincoff HA, Kreissig I, Hahn YS. A temporary balloon buckle for the treatment of small retinal detachments. Ophthalmology 1979;86:586-596.
9. Vrabec TR, Baumal CR. Demarcation laser photocoagulation of selected macula-sparing rhegmatogenous retinal detachments. Ophthalmology 2000;107:1063-1067.
11. Lois N, Wong D. Pseudophakic retinal detachment. Surv Ophthalmol 2003;48:467-487.
12. Schwartz SG, Flynn HW Jr. Primary retinal detachment: Scleral buckle or pars plana vitrectomy? Curr Opin Ophthalmol 2006;17:245-250
13. Schwartz SG, Flynn HW Jr. Pars plana vitrectomy for primary rhegmatogenous retinal detachment. Clinical Ophthalmol 2008;2:1-7 (in press).
14. Cowley M, Conway BP, Campochiaro PA, et al. Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol 1989;107:1147-1151
15. Rodriguez de la Rua E, Pastor JC, Aragon J, et al. Interaction between surgical procedure for repairing retinal detachment and clinical risk factors for proliferative vitreoretinopathy. Curr Eye Res 2005;30:147-153.
16. SPR Study Group. View 2. The case for primary vitrectomy. Br J Ophthalmol 2003;87:784-787.
17. Heimann H, Bartz-Schmidt KU, Bornfeld N et al. Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment: A Prospective Randomized Multicenter Clinical Study. Ophthalmol 2007;114: 2142-2154.



