Ophthalmologists who manage glaucoma know that retinal detachment and glaucoma often coexist. They share several risk factors, including high myopia and prior intraocular surgery. Furthermore, having had a retinal detachment may increase your chances of having glaucoma, and a retinal detachment, by itself, can lead to the disease. Managing glaucoma in these circumstances—especially with surgery—can be challenging. Here, I’d like to offer some strategies that can help that management succeed.
Mechanisms
We’ve known for a while that a history of detachment can be associated with glaucoma. A 1963 study of 530 retinal detachment patients, conducted by Bernard Becker, MD, found that 12.3 percent of them had glaucoma—half of it primary open-angle glaucoma.1 A 1977 survey of 817 patients undergoing primary operations for retinal detachment, conducted by Charles Phelps, MD, and Thomas Burton, MD, found that 9.5 percent of them had glaucoma. (The glaucoma preceded the retinal detachment in 7.3 percent.)2
It’s also clear that a retinal detachment, by itself, can lead to glaucoma. For example, retinal detachment can cause some photoreceptor outer segments to be released into the vitreous fluid; if the anterior hyaloid face has been disrupted by trauma or prior surgery, those segments can migrate into the anterior chamber and clog the trabecular meshwork. However, a more common scenario is that glaucoma develops, or is worsened, as a result of a surgical procedure that was used to repair a retinal detachment.
IOP elevation can occur following pars plana vitrectomy, scleral bucking surgery, injection of intravitreal silicone oil or gas, or even use of postoperative steroids. These can all contribute to pressure elevation, via a variety of mechanisms. Open-angle glaucoma can be triggered by anything that makes the trabecular meshwork not function well, such as trauma, or simply obstructs it, as can happen with emulsified silicone oil or blood cells. Steroids can cause elevated pressure, and volume expansion resulting from overfilling the eye with gas or silicone oil can leave the pressure too high. Inflammation can cause synechiae in the drainage angle, leading to closed-angle glaucoma. Or, injected gas or silicone oil can push the lens and iris forward, causing obstruction of the trabecular meshwork or pupillary block.
Let’s consider the possible reasons each of these retinal detachment re-pair procedures may lead to a rise in pressure.
• Pars plana vitrectomy. The incidence or severity of glaucoma often increases in patients who have undergone pars plana vitrectomy; it’s especially common when such patients have also undergone cataract surgery. Stanley Chang, MD, spoke about this in his 2006 Edward Jackson Lecture reprinted in the American Journal of Ophthalmology.3 He proposed that the mechanism for this could be increased diffusion of oxygen from the vitreous into the anterior chamber, causing oxidative damage to the trabecular meshwork, which would in turn cause reduced aqueous outflow. To my knowledge, this explanation hasn’t been proven, but it’s a reasonable theory.
• Scleral buckle. A tight scleral buckle can compress vortex veins. This can lead to increased venous impedance; congestion, swelling and anterior rotation of the ciliary body; and anterior rotation of the lens-iris diaphragm, with iridotrabecular meshwork apposition. This can lead to angle closure with resulting elevation of the intra-ocular pressure.
A tight scleral buckle can also trigger choroidal effusions. Choroidal ef-
fusions can be transient, resolving without treatment after days or weeks, but they can lead to the development of peripheral anterior synechiae. The result can be acute or chronic elevation of the pressure.
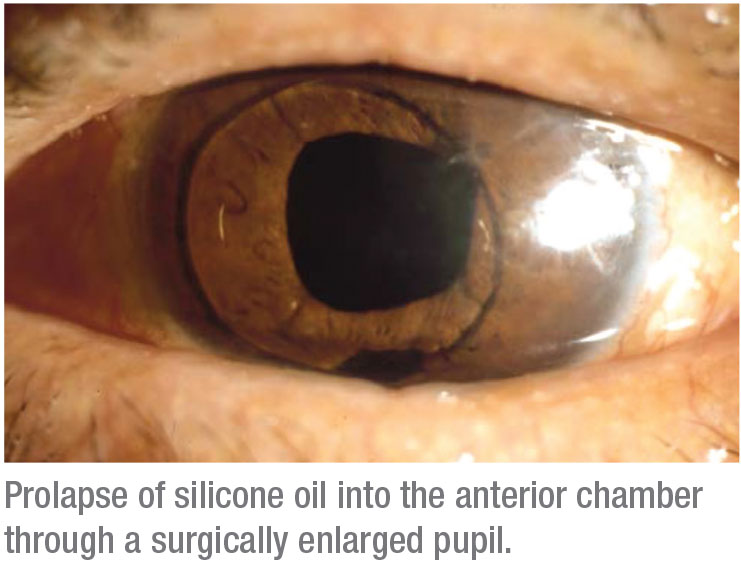 |
• Silicone oil injection. Silicone oil can be problematic, leading to open- or closed-angle glaucoma. If the oil migrates into the anterior chamber, it can obstruct aqueous outflow and/or cause trabecular endothelial damage and dysfunction, even in patients with an open angle. Overfill can cause pupillary block, requiring an inferior iridotomy or iridectomy. It can also lead to closed-angle glaucoma due to anterior displacement of the lens and iris. The angle closure may be asymmetric (more superior than inferior), and it can be exacerbated when the individual is in a supine position.
Clearly, the surgeon needs to avoid overfilling with the oil at the time of surgery; but even with the appropriate amount of oil, emulsified droplets will make their way into the anterior chamber over time and can lead to chronic trabecular obstruction and damage, with intraocular pressure elevation.
• Intravitreal gas injection. Intravitreal gas also can lead to high pressure if you put in too much. The concentration of the gas will determine the rate and magnitude of its expansion; trouble can arise not only from initial overfill, but also if the rate of expansion exceeds the rate of aqueous outflow. As with silicone oil, overfill can cause pupillary block, requiring an iridotomy or iridectomy, or lead to closed-angle glaucoma because of anterior displacement of the lens and iris. Also, as with silicone oil, the resulting angle closure may be asymmetric (more superior than inferior), and it can be exacerbated when the individual is in a supine position.
Managing the Glaucoma
There are a number of ways to ad-dress the elevated pressure:
• First, address any causal mechanism relating to the retinal detachment or its treatment. It’s important to begin addressing the glaucoma by first determining the mechanism that’s caused it, because in many cases it may be possible to reverse that mechanism. For example, if the elevated pressure resulted from the treatment for the retinal detachment, you may be able to mitigate or undo the causal factor. Sometimes removing a little bit of gas will lower the pressure. You may be able to remove a tight scleral buckle or remove silicone oil from the anterior chamber. If steroids are the probable cause, see if you can wait a few weeks, while treating with glaucoma medications, to let the patient taper off of the steroid. The high pressure may resolve and you won’t need to do any glaucoma surgery.
The cause could also be the detached retina itself (rather than the treatment used to address it). If the glaucoma has been triggered by photoreceptor outer segments clogging the trabecular meshwork, reattach the retina; remove any blood (if blood is con-tributing to the problem); and use Avastin or another anti-VEGF agent to address any neovascular component. If appropriate, perform a pars plana vitrectomy.
Admittedly, it’s not always possible to undo an inciting factor; but it’s important to make this your first-line approach to addressing the glaucoma.
• Try medications, topical and otherwise. Once you’ve done what you can to reverse causal mechanisms, move on to medications, laser and surgery to address the elevated pressure. Medications to consider should include: topical medications; miotics (including phospholine iodide) which can be useful in aphakic and pseudophakic patients; oral carbonic anhydrase inhibitors, especially as a temporizing measure; cycloplegia, to minimize anterior rotation of the lens-iris diaphragm; corticosteroids; and intravitreal anti-VEGF injections, if you need to control anterior segment neovascularization.
• Consider using laser. Panretinal photocoagulation, trabeculoplasty and cyclophotocoagulation (transcleral or endoscopic) are all worth considering. Certainly, PRP is a useful treatment for retinal disease, as a means to re-verse neovascularization.
Some people resort to cyclophotocoagulation earlier; others wait and use it as a last resort.
• Incisional procedures. The basic categories here are angle surgery and external drainage procedures. Options include Trabectome and other types of Schlemm’s canal surgery; trabeculectomy with mitomycin-C; variations on trabeculectomy, such as subconjunctival MIGS procedures; and aqueous shunt surgery. Unfortunately, performing glaucoma surgery on a patient who has previously undergone retinal detachment surgery is always a challenge.
Note: It’s worth considering performing glaucoma surgery at the time of vitreoretinal surgery if the patient already had a glaucoma problem before the retinal detachment. That’s especially true if the patient’s IOP isn’t adequately controlled on maximal medication therapy, or if the pressure is adequately controlled on multiple medications, but the patient has moderate to severe disease. Aqueous shunt surgery often is the best procedure in that setting.
Incisional Surgery: Pros & Cons
Let’s look more closely at each incisional surgery option:
 |
• Schlemm’s canal surgeries. Schlemm’s canal surgeries such as an iStent, Kahook blade or Trabectome may be contraindicated because of the presence of silicone oil, angle neovascularization and/or extensive synechial angle closure. However, they can be reasonable options if your target IOP is mid-teens to low 20s; the disc and visual field damage is early to moderate; there’s a fair amount of open angle; and silicone oil is not present in the anterior chamber. Conjunctival scarring doesn’t preclude this option, and it has the advantage that it can be combined with cataract surgery. A potential downside is the possibility of steroid-induced pressure elevation postop.
• Trabeculectomy and its variations. Trabeculectomy is always worth considering when a patient has moderate to advanced disc damage and you need to achieve a very low target IOP, especially if the eye has mobile conjunctiva. However, it can be a less-than-ideal option if you’re dealing with conjunctival scarring from a scleral buckle, for example, especially if the patient has had multiple prior surgeries such as vitrectomy or cataract surgery. Other factors to consider include anterior segment neovascularization; the presence of residual, emulsified silicone oil; and any chronic inflammation that’s present. This type of surgery is also prone to failure following any future surgeries that might become necessary, due to episcleral scarring.
• Aqueous shunt surgery. This is often the preferable choice for addressing elevated pressure in this situation. You can use a tube shunt to manage all degrees of glaucoma severity, from early to advanced, and you can achieve very low pressures (although adjunctive medications are often required). Conjunctival scarring, except in its most severe stages, doesn’t really interfere with doing the surgery or with its outcome. Also, tube shunts are pretty resilient. Even if the patient needs a subsequent operation, tube shunts can continue to work as well as they did when you initially implanted them.
Tube shunt surgery may be contra-indicated if:
— the eye has extensive conjunctival scarring that precludes safe dis-section for this surgery;
— multiple shunts were previously implanted in the eye; or
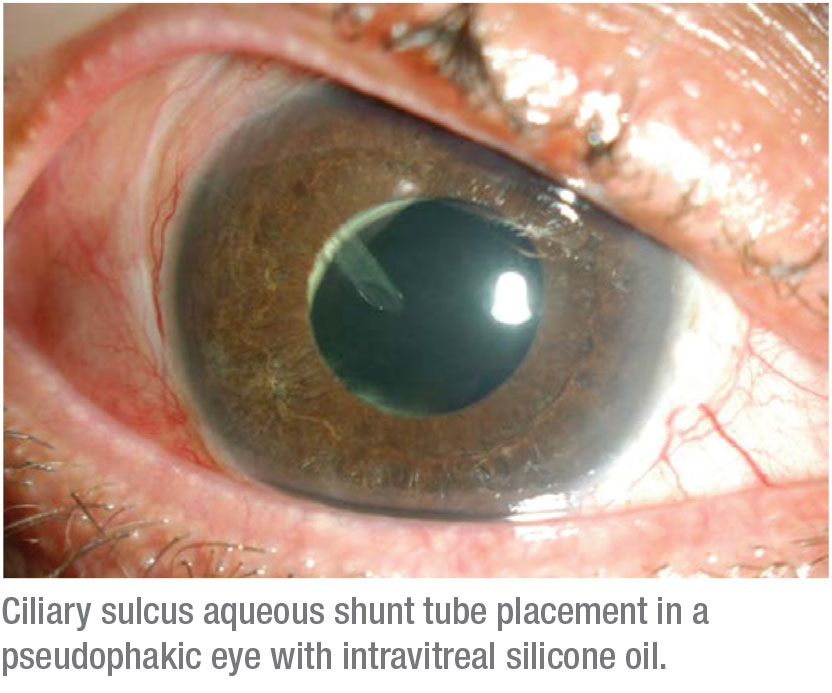
— silicone oil is present and must be retained, and anterior chamber or ciliary sulcus tube placement isn’t possible (e.g., an aphakic eye with extensive anterior synechiae).
• Transscleral cyclophotocoagulation. I usually reserve this option for patients with very poor visual potential, those for whom trabeculectomy or an aqueous shunt are contra-indicated for any of the reasons mentioned above, and those for whom a Schlemm’s canal procedure isn’t appropriate, either because you don’t have an adequate view of the angle, or because you need to achieve a very low IOP. Downsides of this procedure as a stand-alone treatment include the difficulty of titrating the amount of laser administered. There’s a risk of hypotony, which is irreversible after using the laser because there’s nothing to reverse, unlike a tube or trab. Also, the risk of cystoid macular edema and visual problems is greater when large amounts of laser are administered.
One major advantage of this option is that it can be used as an adjunct to a functioning aqueous shunt if the pressure still isn’t low enough. In that situation, you don’t have to do extensive laser and you’ll still improve the outcome. By using a limited amount of laser as an adjunct, you minimize the risk of inflammation and CME, and you may avoid the need to implant an additional shunt.
When Implanting a Shunt
Implanting an aqueous shunt in this situation requires careful preoperative assessment. For example, you need to consider the number, type and location of prior surgeries and the amount and location of conjunctival scarring. Are you dealing with scleral ectasia? Will you need to work around a pre-existing filtering bleb or aqueous shunt? If you’re faced with a scleral buckle, what type of tube shunt should you use? Where should you place it? Is the conjunctiva mobile? What’s the status of the conjunctival fornices? Are they foreshortened? Do you see symblepheron formation? Is the patient phakic, aphakic or pseudophakic? What’s the configuration of the anterior chamber and the anterior chamber angle?
• Placing the plate. If the patient is phakic or pseudophakic, it’s preferable to place the plate in the superotemporal or inferonasal quadrant. If the patient is aphakic—especially if the patient has a large pupil—the inferonasal quadrant is often best if gas or silicone oil are pre-sent, or may be required.
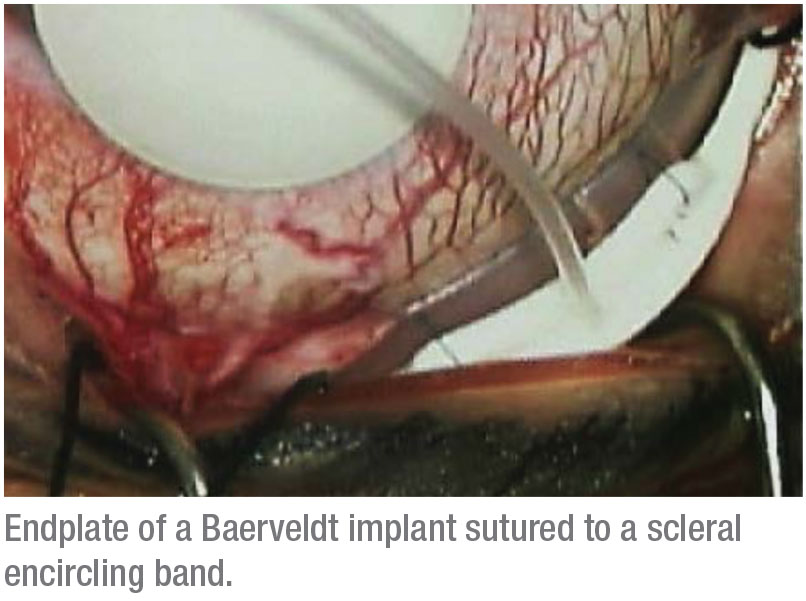
• Working with a scleral buckle. When dealing with an existing scleral buckle, or when putting on a buckle at the time of the glaucoma surgery, a low-profile implant like a Baerveldt is a good choice. Its thin and flexible plate is preferred for inserting over a scleral encircling band. It’s best to suture it directly to the upper surface of the scleral buckling element. If adjacent rectus muscles can be isolated, you can place the wings of the Baerveldt plate beneath the muscles. (This arrangement works well with narrow, low-profile encircling elements such as the MIRA #40 band.) You can place the plate above the muscles if the degree of scarring is too extensive; you also have the option of trimming the Baerveldt plate if extensive scarring makes placement difficult.
It’s helpful to know the type and location of the scleral buckling element you’re dealing with. Is it a complete encircling band, or just a segmental buckle? Has it migrated forward, or is it 10 mm back from the limbus? That information will help determine where and how you’ll need to place your glaucoma drainage device. If it’s 10 mm or more back, you can suture it to the encircling band. If the band has migrated forward and it’s 5 mm or less from the limbus, you’re probably going to have to secure your plate behind the buckle. (Often you can determine this before you get to the OR.)
The anterior edge of the plate should be 9 to 12 mm posterior to the limbus and sutured directly to the encircling band when possible. Avoid attempting to place it beneath the encircling band; the sclera is often very thin under the band, especially if the band has been on the eye long-term, and scleral perforation is a risk.
• Placing the tube. You can’t place the tube between the iris and the cornea if the iris is pulled up to the cornea because of synechiae, which is not uncommon in these complex eyes. In that situation you want to put the tube either in the ciliary sulcus or through the pars plana, behind the lens implant and into the vitreous cavity. If it goes into the vitreous cavity, you’ll need to have removed all of the vitreous gel to avoid occlusion of the tube. In pseudophakic patients, you can put the tube in the sulcus without doing a vitrectomy. (A more posterior location of the tube entry site may facilitate conjunctival closure.)
Tubes and Silicone Oil
 |
When implanting a tube shunt in an eye that either already contains silicone oil, or into which you’ll be placing silicone oil, you need to ask a number of questions: Is the patient phakic, aphakic or pseudophakic? How high is the intraocular pressure? Will you be removing the oil? (If so, you can put the tube in the vitreous cavity. If the oil has to stay in, you can’t put the tube back in the posterior segment.) Can you wait for silicone oil removal at a future date, or do you need to put the tube in right away to reduce the pressure immediately? What’s the status of the corneal endothelium? What’s the status of the iris (pupil size, and location and size of iridectomies)? Is there silicone oil in the anterior chamber? If so, does it need to be re-moved prior to tube insertion?
A few specific issues to consider:
• Consider a two-stage procedure. If you’re going to remove the oil in six weeks, you can put the plate on at the time of surgery but hold off on inserting the tube. In a few weeks, when you go back to take the oil out, you can insert the tube.
Of course, in some patients, the oil never comes out. If you believe that’s going to be the case with your patient, waiting to insert the tube wouldn’t be a great strategy. You could still do the tube shunt surgery in two stages, but you won’t be able to put the tube into the vitreous cavity. You’d wind up having to put the tube in the front of the eye (if there was a place for it).
• Don’t allow anterior prolapse of the oil. You have to keep the tip of the tube away from the silicone oil, because if the oil gets into the tube, the implant won’t work well. It also could drain oil out of the eye, which would interfere with good retinal tamponade. To prevent anterior prolapse of the oil, you need to avoid abrupt decompression of the eye. You have to be careful about how you put in the tube, so the pressure doesn’t drop precipitously. Intraoperatively, while the patient is supine, viscoelastic may help prevent forward movement of the silicone oil.
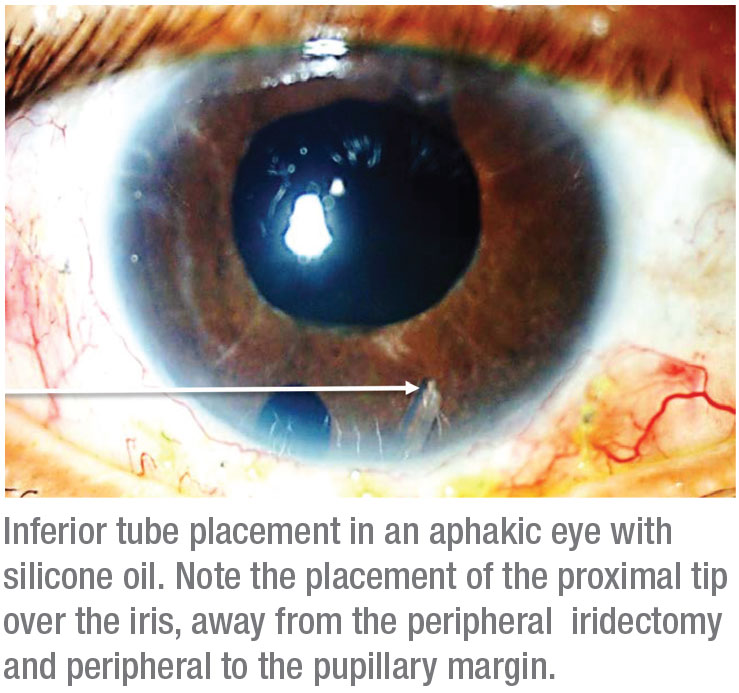
• Avoid allowing the tube tip to contact the silicone oil. For aphakic patients, keep the tip of the tube peripheral to the pupillary margin. The iris can act as a shield that protects the tube tip from the silicone oil. If the tube tip extends beyond the pupillary margin, the oil will have access to the tip of the tube. Avoid placing the proximal tube tip over iridectomies or iris defects.
• If you’re doing the tube implant and retinal surgery concurrently, put the tube in first and infuse the oil last. If the oil goes in first and you make a hole to put the tube in, the pressure will often drop and oil will come forward into the front of the eye. Once that happens, it can be problematic to get the oil out of the anterior chamber.
• A Baerveldt shunt may be preferable to an Ahmed. In this situation I like to use non-flow-restricted devices. The Ahmed valve is a little bit unpredictable, and sometimes you wind up with a really low pressure. In an eye with silicone oil, that can be a problem.
For that reason I prefer to use a tube that’s completely ligated. You can use any of a variety of methods to control the pressure for the first few weeks, or you can do nothing and manage the patient with medications until the plate becomes encapsulated. Once the plate’s encapsulated, you can open the tube.
 |
One way to control the pressure during the first few weeks is to create an “orphan trabeculectomy,” done without using mitomycin-C. Without the mitomycin, a trabeculectomy in this setting will generally fail with-in a few weeks. During that time the glaucoma implant plate is encapsulating. You can laser the stitches selectively, if necessary, to get a gradual reduction in pressure. (You don’t want an abrupt pressure drop, or the oil might come forward.)
Eventually the trabeculectomy will scar over and fail, but by then the tube is ready to take over controlling the pressure. This approach isn’t used often because it’s a significant amount of additional surgery compared to just fenestrating the tube, but in some patients, it may give you a little bit more control.
• If inserting the tube in the presence of silicone oil, use viscoelastic rather than an infusion to keep the chamber from shallowing. Using an infusion as an anterior chamber maintainer when inserting a tube isn’t a good idea if there’s pre-existing silicone oil in the eye. The fluid can end up going behind the oil and pushing it forward. However, you can use some viscoelastic to keep the chamber from abruptly shallowing as you enter the eye with the needle and try to position the tube.
• Controlled tube ligature release is helpful. I usually release the ligature at three to four weeks. You want to be careful about doing it sooner because of the risk that the plate isn’t fully encapsulated. However, you don’t want to wait much beyond the five-week mark. If you tie the tube with a 7-0 vicryl suture, by six weeks postop it will dissolve, opening the tube. If that happens between visits, the result will probably be an abrupt drop in pressure, often down to the single digits, as the fluid rushes out of the eye and fills the reservoir. Among other hypotony-related problems, that may cause the silicone oil to move forward into the anterior chamber.
To avoid that, I do a planned ligature release at three to four weeks. I discontinue aqueous suppressant medications several days beforehand, if the IOP isn’t terribly high. Then I inject some viscoelastic into the anterior chamber at the slit lamp before I laser the ligature, to prevent hypotony and anterior chamber shallowing. Following ligature release, additional viscoelastic can be injected to maintain the anterior chamber depth and keep the pressure at a physiologic level.
Making the Best of It
The key point I hope you’ll take home from this discussion is that glaucoma often is associated with retinal detachment. Sometimes it’s pre-existing; sometimes it’s related to the detachment itself, or to its treatment.
When you need to address elevated pressure in this situation, surgery may be required. If that’s the case, Schlemm’s canal surgeries may work in patients with early-to-moderate damage. If you need a more substantial drop in pressure, trabeculectomy is possible, but it’s technically challenging in these patients. In most cases, an aqueous tube shunt is a better solution. REVIEW
1. Becker B, in discussion, Smith JL. Retinal detachment and glaucoma. Trans Am Acad Ophthalmol Otolaryngol 1963;67:731-732.
2. Phelps CD, Burton TC. Glaucoma and retinal detachment. Arch Ophthalmol 1977;95:3:418-22.
3. Chang S. LXII Edward Jackson lecture: Open-angle Glaucoma After Vitrectomy. Am. J. Ophthamol 2006;141:1033-1043.



