Presentation
A 79-year-old Caucasian female with 10 days of progressive double vision and left upper eyelid droop presented for ophthalmic evaluation. She denied flashes, floaters, jaw claudication, fever, unintentional weight loss or photophobia. Systemic review of symptoms was notable for 1.5 years of intermittent confusion, speech problems, severe headaches and memory deficits.
History
The patient denied any past ocular history. She had a significant past medical history of diverticulitis, polymyalgia rheumatica, hypertension and subdural hematoma. Her oncologic history was notable for breast cancer (stage 1, HER-2 negative, ER positive) for which she underwent lumpectomy and external beam radiation 10 years prior to presentation. She also had a mature cystic teratoma removed by hysterectomy and oophorectomy two years prior. Over the course of the past year, she had multiple admissions to an outside hospital for neurologic symptoms, including aphasia and seizures. Imaging at that time revealed progressive pachymeningitis and several pulmonary nodules with abnormal 18F-fluorodeoxyglucose avidity on PET/CT concerning for metastasis. A previous lumbar puncture showed elevated white blood cell count and protein with negative cytology. She later underwent lung biopsy, which was negative for malignancy but showed acute and chronic inflammation. Notably, the patient had progressively increasing Westergren erythrocyte sedimentation rate (WESR) levels from 31 mm/hr to a peak of 92 mm/hr, at which point she was started on oral dexamethasone for palliative treatment of leptomeningeal disease. Rheumatologic work-up, including anti-nuclear antibody (ANA) panel, immunoglobulin G4 (IgG4) and rheumatoid factor (RF) were unremarkable.
The patient was a non-smoker but consumed approximately five glasses of wine per week. Family history was significant for colon cancer, stroke and Parkinson’s disease. Her medications included: alprazolam and bultabital-caffeine-acetaminophen as needed, daily aspirin, dexamethasone 2 mg twice daily, levetiracetam, metoprolol and rosuvastatin.
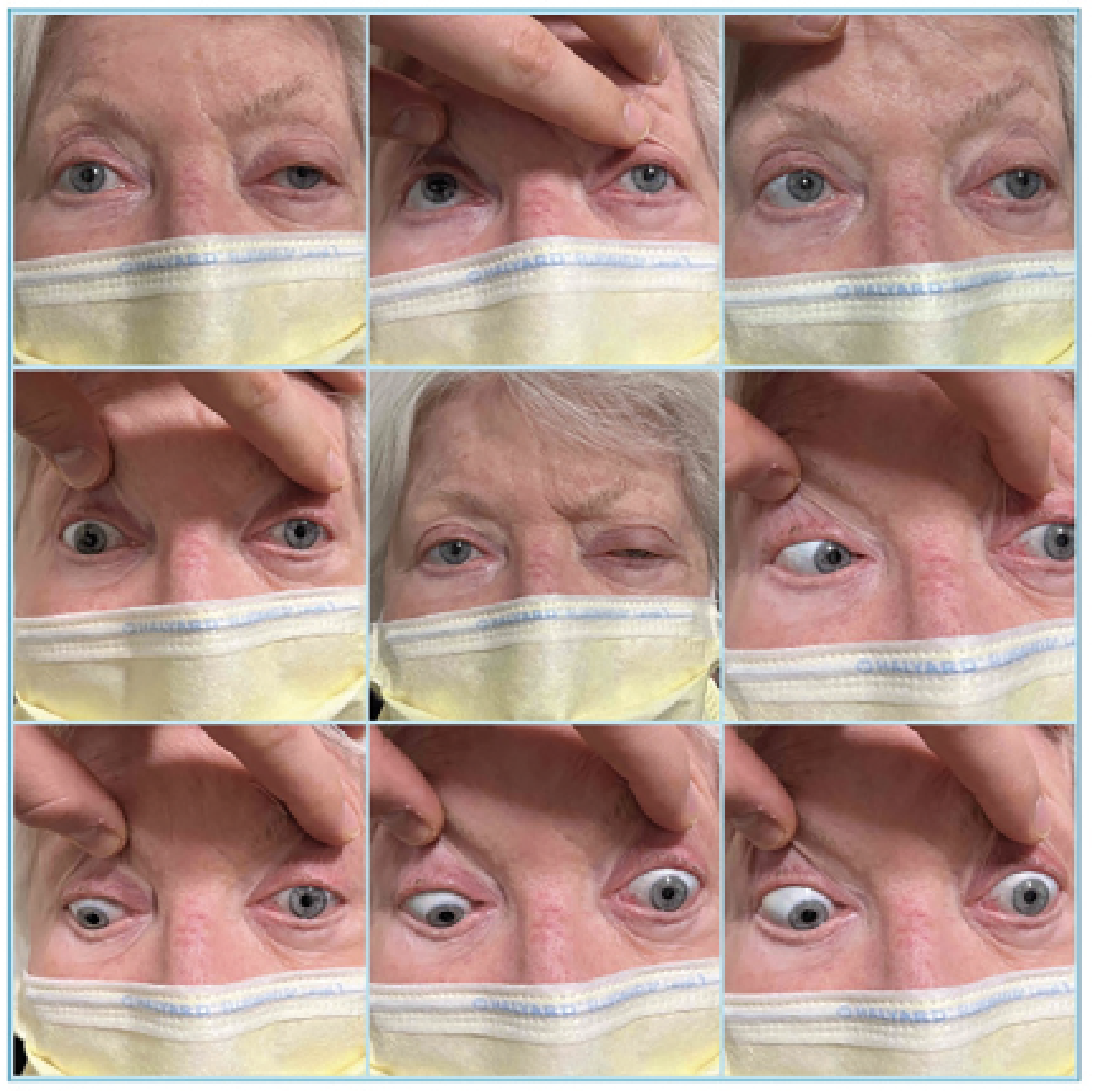 |
| Figure 1. Left ptosis and bilateral external ophthalmoplegia. |
Examination
The patient’s vital signs were within normal limits. Best-corrected visual acuity was 20/30 in the right eye and 20/60 in the left. Intraocular pressures were within normal range. There was a 1+ relative afferent pupillary defect in the left eye. Confrontation visual fields were full. Ishihara color plates were 6/8 in the right eye and 2/8 in the left eye. Extraocular motility was globally restricted in the left eye and to a lesser degree on the right (Figure 1). Hertel exophthalmometry with a base of 94 mm revealed measurements of 18 mm OD and 22 mm OS. There was frontalis recruitment bilaterally with left upper eyelid ptosis and decreased levator function.
The anterior segment examination was largely unremarkable apart from bilateral nuclear sclerosis and posterior subcapsular cataracts. Dilated fundus examination of the right eye showed a normal optic disc and macula with a sclerotic vessel superiorly and pigmented cobblestone degeneration temporally. The left eye had changes of moderate non-proliferative diabetic retinopathy and scattered peripheral drusen with normal optic disc and macula.
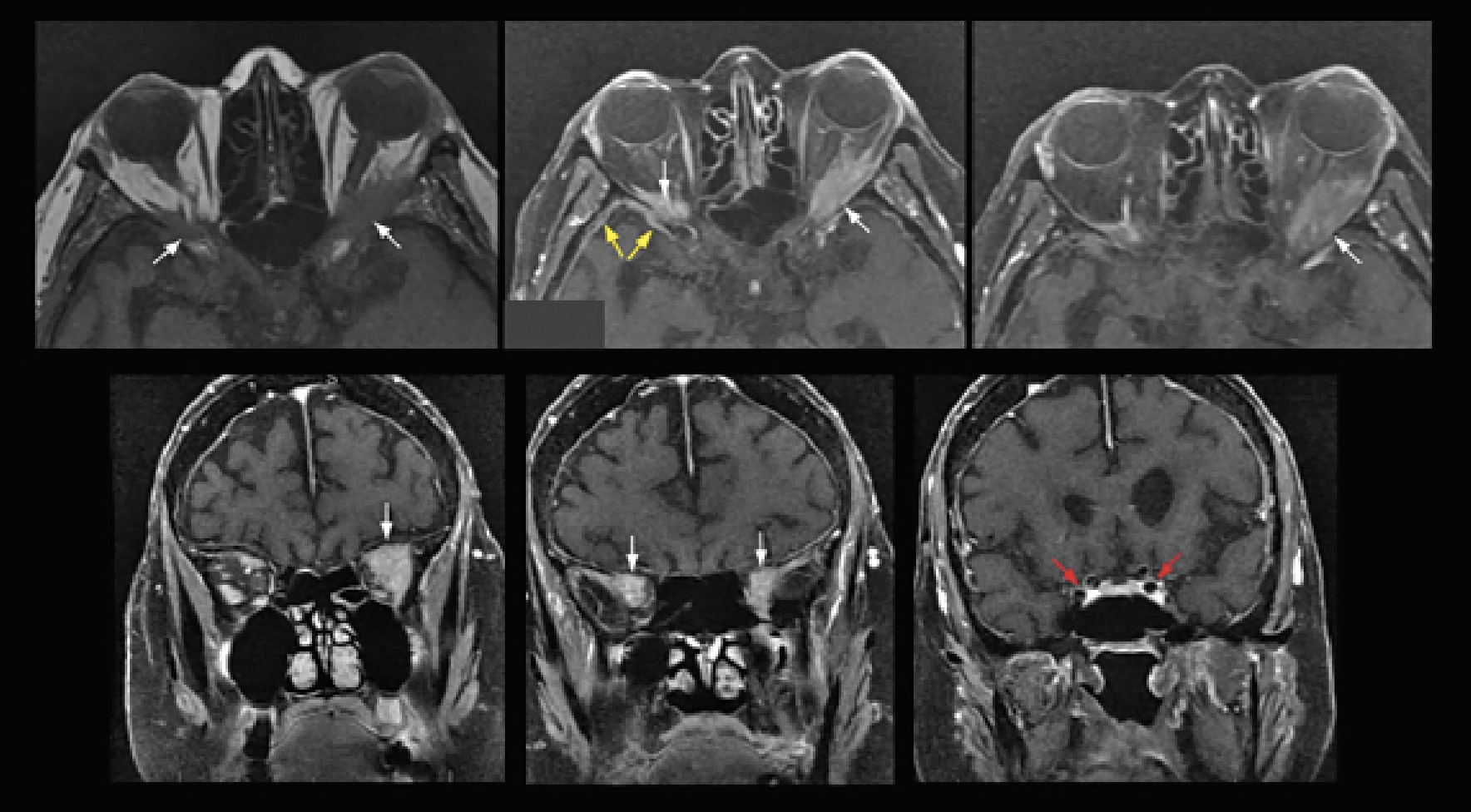 |
| Figure 2. Axial and coronal cuts of T1-weighted MRI (upper left, pre-contrast; remaining images are post-contrast), showing pachymeningeal enhancement (yellow arrows) and bilateral orbital masses (white arrows), left greater than right. Note the lack of cavernous sinus involvement (red arrows). |
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
Brain and orbital MRI with and without contrast revealed masses at the bilateral orbital apices (Figure 2). T1-weighted post-contrast images also showed an enhancing mass in the left jugular foramen in addition to the previously described pachymeningeal enhancement. Diffusion weighted imaging revealed an acute infarct in the right postcentral gyrus and restricted diffusion within the orbital lesions (Figure 3). The updated differential diagnosis included primarily inflammatory and neoplastic etiologies, including metastatic breast carcinoma.
Laboratory work-up included angiotensin-converting enzyme, complement 3/4 levels, lactate dehydrogenase, thyroid stimulating hormone and immunoglobulin, anti-neutrophil cytoplasmic antibody (ANCA), serum and urine protein electrophoresis, and anti-ribonucleoprotein, all of which were negative. WESR and C-reactive protein were within normal limits. ANA was positive with a 1:320 titer.
The patient was admitted for multi-disciplinary management with neurology, rheumatology and medical oncology. She subsequently underwent left orbitotomy for exploration and biopsy. Pathology revealed dense fibrosis, fat necrosis and basophilic necrosis along small vessels without involvement of medium- or large-sized vessels (Figure 4). Special stains for microorganisms were negative. The diagnosis of small vessel vasculitis was later supplemented with anti-proteinase 3 (pr3) antibody positivity, consistent with granulomatosis with polyangiitis (GPA). She was treated with pulsed dose IV methylprednisolone for three days and discharged with a prednisone taper and biweekly rituximab infusions for one month.
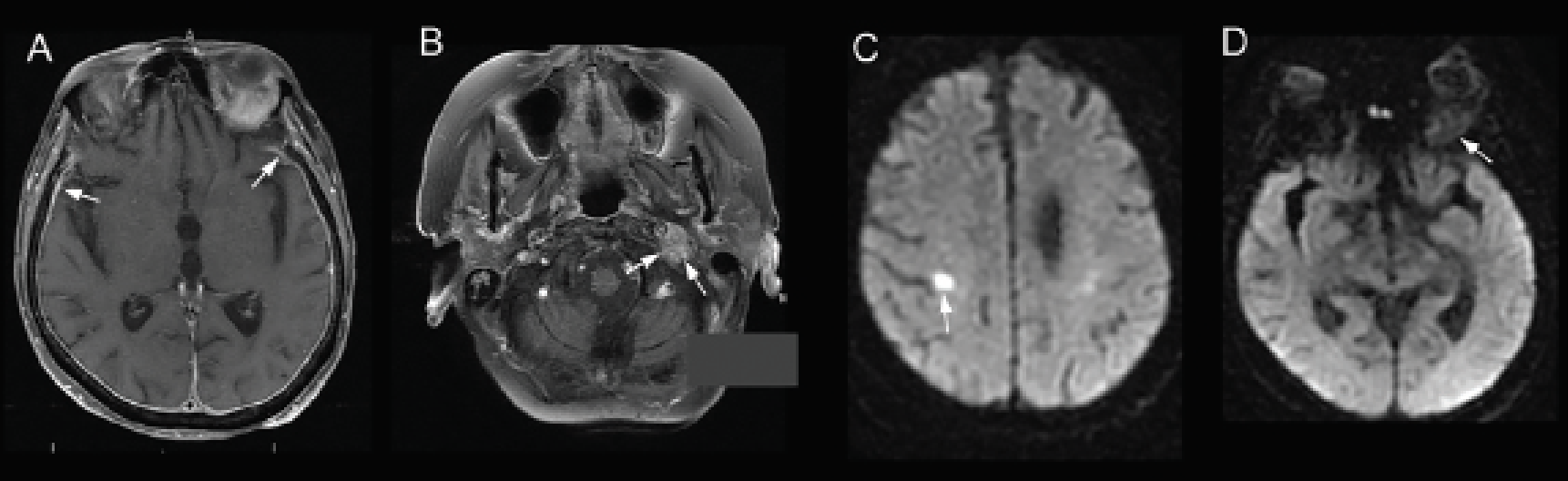 |
| Figure 3. T1-weighted, post-contrast MRI showing (A) pachymeningeal enhancement (arrows) and (B) mass encasing the left jugular foramen (arrows). Diffusion weighted imaging showing (C) acute infarct of right precentral gyrus (arrow) and (D) restricted diffusion within the left orbital lesion (arrow). |
Discussion
GPA is a rare small-vessel necrotizing vasculitis. It is one of three ANCA-associated vasculitides; the other two are microscopic polyangiitis (which occurs exceedingly rarely in the orbit) and eosinophilic granulomatosis with polyangiitis. Although its pathogenesis is not fully understood, it’s presumed that ANCA in GPA stimulates activation of neutrophils with proteinase 3 antigen, which induces degranulation that affects endothelial cells, resulting in vessel wall injury, and promotes T-cell activation triggering macrophage maturation and formation of granulomas.1 There also appears to be complex gene-environment interactions with numerous implicated genes, including HLA-DPB1, HLA-DPA1, SEMA6A, CTLA4, PTPN22 and others.2-4
GPA classically presents as a triad of respiratory tract inflammation, pulmonary infiltrates and glomerulonephritis. Ocular and orbital involvement is observed in more than half of patients with GPA and isn’t uncommonly the presenting feature. Ocular manifestations are wide-ranging and may include exophthalmos, diplopia, ocular pain, ophthalmoplegia, cicatricial conjunctivitis, peripheral ulcerative keratitis, retinal vascular occlusion and, infrequently, uveitis.5-7 Central nervous system involvement in GPA is rare, occurring in about 7 to 11 percent of cases, and manifests as three distinct clinical patterns: cerebral vasculitis; pituitary gland involvement; and/or chronic hypertrophic pachymengintis.8 Lumbar puncture in these cases typically reveals pleocytosis and elevated protein concentrations.9,10
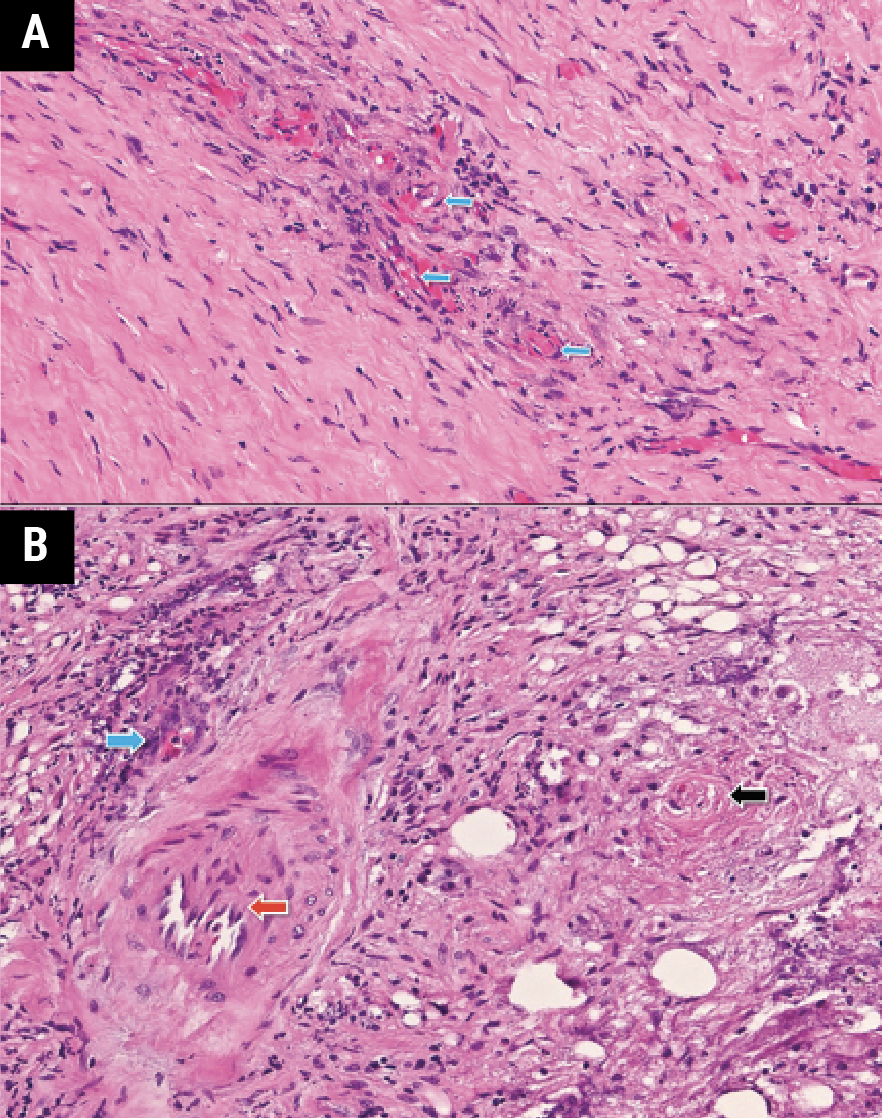 |
| Figure 4. H&E stain of left orbital mass biopsy showing (A) small vessel vasculitis (blue arrows) and (B) basophilic necrosis near vascular endothelium (blue arrow), unaffected large vessel (red arrow), and occluded small vessel (black arrow). |
Serologic studies have varying utility in the diagnosis of orbital inflammation, typically requiring high disease activity to yield a positive result.11 ANCA appears to be a marker of disease activity, and in the limited form of sino-orbital GPA up to 60 percent of cases may have negative ANCA immunofluorescence.5,12,13 Moreover, there’s a variability in how these assays are performed. The most widely accepted approach is to test myeloperoxidase-ANCA (MPO) and pr3-ANCA by ELISA only after positive screen with immunofluorescence.14 However, the International Consensus Statement recommends that optimally, concurrent testing be done in all patients suspected of having ANCA-associated vasculitis.15 Although quite rare, there have been instances of IF-negative, MPO/pr3-ANCA positive vasculitis.16 To optimize cost-effective testing and avoid excessive false positives, clinical suspicion of ANCA-associated vasculitis should guide ELISA testing when immunofluorescence is unrevealing.17
Biopsy remains the gold standard in diagnosis. Clinical improvement with high-dose corticosteroids in both benign and malignant disease can lead to an incorrect empiric diagnosis of idiopathic orbital inflammation and delay the diagnosis of insidious neoplastic processes.11 It’s therefore important to pursue biopsy of surgically accessible tissue prior to an empiric corticosteroid trial, especially when serology and imaging are equivocal. In this case, one of the leading diagnoses was bilateral orbital metastases from breast carcinoma; metastatic breast carcinoma to the orbits is bilateral in about 20 percent of cases.18 Definitive diagnosis was critical for correct management. The diagnosis of ANCA-associated vasculitis requires a combination of careful history-taking, thoughtful laboratory testing, radiographic evidence and histopathologic findings. Once a fatal disease with median survival of five months, GPA now has an excellent prognosis, with 95-percent survival at five years and 80 percent at 10 years due to a combination of glucocorticoid and immunomodulatory therapy.6 Recent studies have recommended rituximab as the mainstay of treatment.19
This case involved a unique presentation of bilateral orbital apical masses preceded by progressive pachymeningitis in a patient with a prior history of breast cancer. It’s further distinguished by the serologic findings of IF-negative but pr3-ANCA antibody positive vasculitis. The constellation of symptoms could have easily been mistaken for metastases or several separate processes, but with a thorough work-up and ultimately tissue biopsy, the diagnosis of GPA was made, with prompt initiation of appropriate treatment.
1. Csernok E, Gross WL. Current understanding of the pathogenesis of granulomatosis with polyangiitis (Wegener’s). Expert Rev Clin Immunol 2013;9:7:641-8.
2. Xie G, Roshandel D, Sherva R, et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum 2013;65:9:2457-68.
3. Borgmann S, Endisch G, Urban S, et al. A linkage disequilibrium between genes at the serine protease inhibitor gene cluster on chromosome 14q32.1 is associated with Wegener’s granulomatosis. Clin Immunol 2001;98:2:244-8.
4. Jagiello P, Aries P, Arning L, et al. The PTPN22 620W allele is a risk factor for Wegener’s granulomatosis. Arthritis Rheum 2005;52:4039–4043.
5. Tarabishy AB, Schulte M, Papaliodis GN, et al. Wegener’s granulomatosis: Clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Surv Ophthalmol 2010;55:5:429–444.
6. Sfiniadaki E, Tsiara I, Theodossiadis P, et al. Ocular manifestations of granulomatosis with polyangiitis: A review of the literature. Ophthalmology and therapy 2019;8:2:227.
7. Tarabishy AB, Schulte M, Papaliodis GN, et al. Wegener’s granulomatosis: Clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Survey of Ophthalmology 2010; 55:5:429-444.
8. Seror R, Mahr A, Ramanoelina J, et al. Central nervous system involvement in Wegener granulomatosis. Medicine (Baltimore) 2006;85:1:53-65.
9. Nagashima T, Maguchi S, Terayama Y, et al. P-ANCA-positive Wegener’s granulomatosis presenting with hypertrophic pachymeningitis and multiple cranial neuropathies: Case report and review of literature. Neuropathology 2000;20:23-30.
10. Newman NJ, Slamovits TL, Friedland S, et al. Neuro-ophthalmic manifestations of meningocerebral inflammation from the limited form of Wegener’s granulomatosis. Am J Ophthalmol 1995;120:5:613-21.
11. Mombaerts I, Rose GE, Garrity JA. Orbital inflammation: Biopsy first. Surv Ophthalmol. 2016;61:5:664-9.
12. Woo TL, Francis IC, Wilcsek GA, et al. Australasian orbital and adnexal Wegener’s granulomatosis. Ophthalmology 2001;108:9:1535-1543
13. Tan LT, Davagnanam I, Isa H, et al. Clinical and imaging features predictive of orbital granulomatosis with polyangiitis and the risk of systemic involvement. Ophthalmology 2014;121:6:1304-9.
14. Stone JH, Talor M, Stebbing J, et al. Test characteristics of immunofluorescence and ELISA tests in 856 consecutive patients with possible ANCA-associated conditions. Arthritis Care Res (Hoboken) 2000;13:424–34.
15. Savige J, Gillis D, Benson E, et al. international consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999;111:4:507-13.
16. Rao DA, Wei K, Merola JF, O’Brien WR, Takvorian SU, Dellaripa P.F, Schur PH. Myeloperoxidase-antineutrophil Cytoplasmic Antibodies (MPO-ANCA) and Proteinase 3-ANCA without Immunofluorescent ANCA Found by Routine Clinical Testing. The Journal of rheumatology 2015;42:5:847–852.
17. Tsiveriotis K, Tsirogianni A, Pipi E, Soufleros K, Papasteriades C. Antineutrophil cytoplasmic antibodies testing in a large cohort of unselected greek patients. Autoimmune Diseases 2011;626495.
18. Goldberg RA, Rootman J, Cline RA. Tumors metastatic to the orbit: A changing picture. Surv Ophthalmol 1990;35:1-24.
19. Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;15:363:3:221-32



