Presentation
A 66-year-old female presented to Wills Eye Hospital with recurrent right eye pain and photophobia four months after combined ab interno canaloplasty with the Omni system, Schlemm’s canal microstent implantation (Hydrus Microstent), and cataract surgery by an outside ophthalmologist. Ocular history was remarkable for primary open angle glaucoma managed with topical glaucoma medications in both eyes.
After the cataract surgery, she developed persistent iritis in the right eye that was treated with topical steroids. Over the next few months, she tried to taper off topical steroid multiple times without success. Steroid response was noted with the patient’s intraocular pressure in the right eye increasing to 26 mmHg while on topical prednisolone acetate.
History
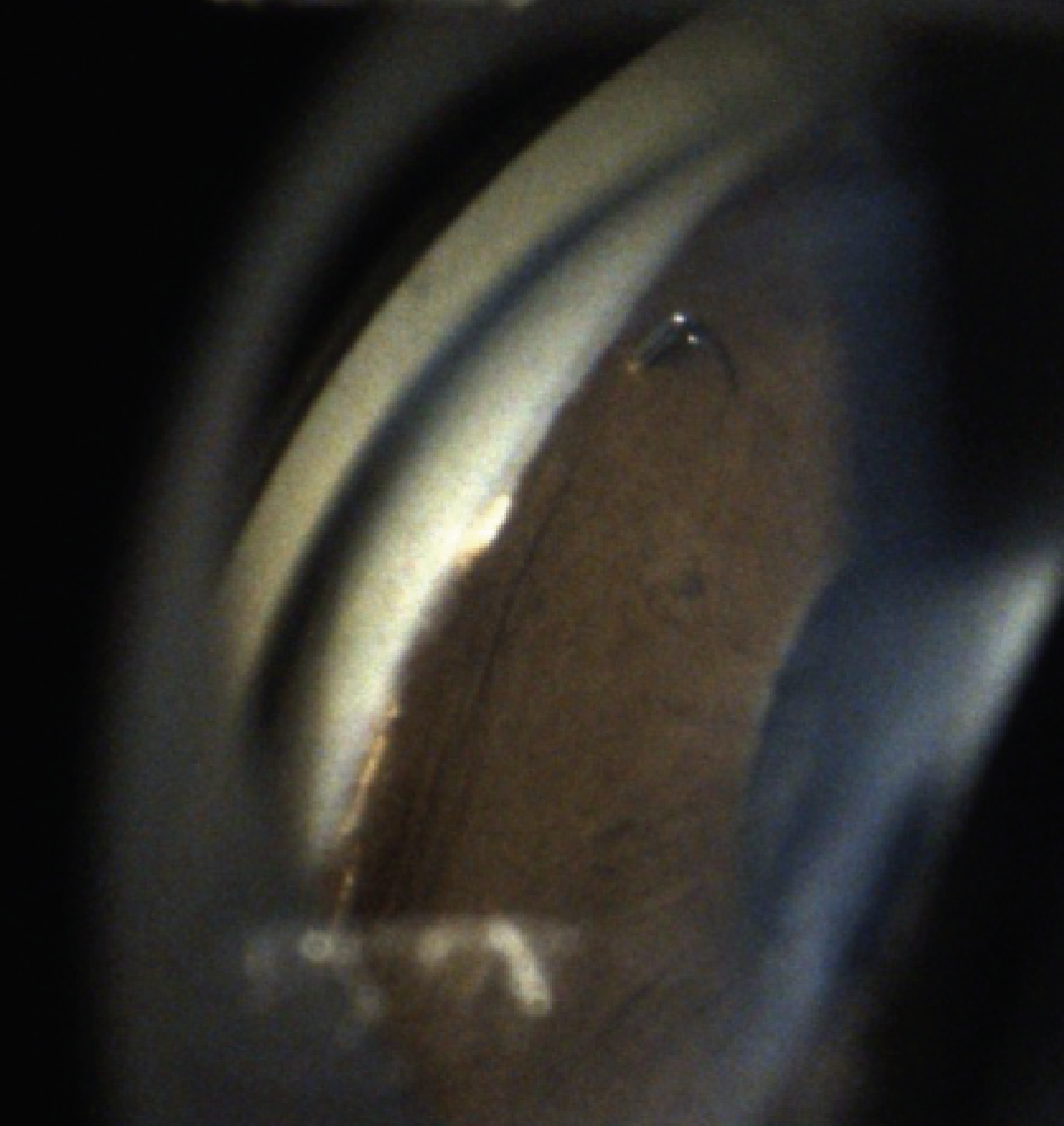 |
| Figure 1. A gonioscopic photograph of the right eye demonstrating the Schlemm’s canal microstent inlet incarcerated in peripheral anterior synechiae. |
Past medical history included osteoarthritis and hypertension. In addition to the mentioned surgery, her POAG had been treated with selective laser trabeculoplasty in both eyes. Family and social histories were non-contributory. She reported no drug allergies. Oral medications included amlodipine, atorvastatin and alendronate. At the time of presentation her topical medications included brimonidine/timolol two times daily in the right eye, dorzolamide two times daily in the right eye, bimatoprost every evening in both eyes and loteprednol four times daily in the right eye.
Examination
Ophthalmic examination revealed best-corrected visual acuity of 20/30 in both eyes. The pupils were equally round and reactive to light with no relative afferent pupillary defect. Her IOP was 18 mmHg OD and 13 mmHg OS. Confrontation visual fields and extraocular motility were full OU. Anterior segment examination of the right eye was notable for diffuse corneal endopigment, superonasal and inferotemporal limbal relaxing incisions, trace pigmented cell in the anterior chamber, a one-clock-hour inferior iridodialysis and a PCIOL that appeared to be in the capsular bag.
Anterior segment examination OS was notable for 2+ nuclear sclerotic cataract. Gonioscopy OD showed an inferior iridodialysis and a nasal Schlemm’s canal microstent with surrounding peripheral anterior synechiae and protrusion of the stent inlet into the anterior chamber. The microstent inlet was noted to be partially incarcerated in iris tissue (Figure 1). The iridocorneal angles were otherwise noted to be open to the scleral spur OU. Dilated fundus examination showed a cup-to-disc ratio of 0.65 in the right eye and 0.45 in the left eye without disc notching, pallor or hemorrhage.
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
 |
| Figure 2. Ultrasound biomicroscopy demonstrating nasal microstent, and inferior iridodialysis, and well-positioned PCIOL in the capsular bag. |
Work-up, Diagnosis and Treatment
Optical coherence tomography of the peripapillary retinal nerve fiber layer demonstrated inferotemporal thinning OD and no thinning OS. OCT of the macula was within normal limits OU. Automated perimetry with the Octopus perimeter (Haag-Streit) revealed an inferior nasal step OD and non-specific defects OU. Ultrasound biomicroscopy was notable for an inferior iridodialysis, an open iridocorneal angle, a PCIOL centered in the capsular bag and a nasal Schlemm’s canal microstent OD (Figure 2).
The differential diagnosis for this case includes postoperative rebound iritis, uveitis-glaucoma-hyphema syndrome from microstent malposition, and infectious or autoimmune etiologies or anterior iritis. Given the posterior malposition of the Schlemm’s canal microstent, the leading diagnosis was stent-iris chafing causing UGH syndrome.
Multiple additional attempts were made to taper topical steroids OD and a topical non-steroidal anti-inflammatory drop was added OD. However, the patient experienced rebound of pain and photophobia with each attempt to discontinue topical steroids. Subsequent examinations of the right eye revealed IOPs ranging from 18 to 31 mmHg and persistent trace pigmented cells in the anterior chamber despite use of maximum tolerated topical glaucoma medications.
Given the patient’s persistent anterior uveitis and uncontrolled IOP with topical steroid use, the patient was offered removal of the malpositioned Schlemm’s canal microstent with possible iris repair OD. The possibility of concurrent glaucoma filtering surgery was also discussed, but the patient elected to proceed with microstent removal alone to see if her IOP would improve with treatment of the stent-iris chafing.
Microstent removal was performed under direct gonioscopic visualization. Viscoelastic was used to free peripheral anterior synechiae from the microstent inlet (Figure 3). A microvitreoretinal blade was then used to lyse remaining synechiae around the microstent scaffold so that it was no longer incarcerated in iris tissue (Figure 3). MicroSurgical Technology (MST) forceps were then used to remove the microstent from the eye with a resulting 3-clock-hour goniotomy (Figure 3). No additional iridodialysis was created during this procedure.
 |
| Figure 3. Intraoperative photographs demonstrating viscodissection, lysis of iris adhesions with an MVR blade, and removal of the microstent from the anterior chamber. |
Postoperatively, the patient’s vision improved to 20/20 OD, and she was able to taper topical lotedprednol to one drop every 48 hours, but her IOP remained uncontrolled, with intermittent elevations as high as 25 mmHg OD. She was referred to a uveitis specialist for consideration of other etiologies of anterior uveitis, but no additional workup was recommended. Repeat OCT RNFL demonstrated progressive thinning inferiorly and superiorly in the right eye. Her visual fields remained stable OU. Given the concern for glaucomatous progression, the patient was recommended to undergo filtering surgery in the right eye.
Discussion
The Hydrus Microstent (Alcon/Ivantis) is an ab interno trabecular microbypass stent that received FDA approval in August 2018 for implantation during phacoemulsification in cases of mild to moderate primary open-angle glaucoma. The biocompatible titanium and nickel alloy stent is 8 mm in length and 290 µm in diameter, featuring three posterior windows and an inlet in the anterior chamber. The HORIZON study demonstrated its superiority over phacoemulsification alone in a prospective cohort study of 546 patients with mild to moderate POAG, showing greater improvements in unmedicated IOP and a reduction in the number of hypotensive eye drops at both 24 and 60 months.1-3
Ab interno Schlemm’s canal viscodilation (Omni/Visco360, Sight Sciences) is another minimally invasive glaucoma surgery used for treating mild to moderate primary open-angle glaucoma in conjunction with phacoemulsification. In a study of 106 eyes, there was a reduction in IOP and the number of hypotensive eye drops with this technique.4 The combined use of the Schlemm’s canal microstent with additional canaloplasty during cataract surgery may lead to further reductions in IOP. However, studies have inconsistently demonstrated its benefit, with one study showing no change in medicated IOP but a possible reduction in the number of ocular hypotensive medications.5 Other ab interno trabecular microbypass stents (iStent) combined with canaloplasty at the time of phacoemulsification also failed to conclusively demonstrate a benefit in IOP or hypotensive eye drop reduction over stent placement without canaloplasty during cataract surgery.6
Schlemm’s canal microstent malposition has been reported in the literature as a rare cause of UGH syndrome. In two case reports, removal of the device was necessary for the resolution of intraocular inflammation.7,8 Notably, in both reports, patients required further filtering surgery for adequate IOP control.7-8 In the five-year follow-up of the HORIZON study, device malposition was noted in 1.4. percent of cases, with inflammation requiring steroids for more than a month in 5.9 percent of cases.1-3 However, none of the devices was noted to have migrated after implantation or ultimately require explantation.3 A review of the FDA Manufacturer and User Facility Device Experience (MAUDE) database between 2009 and 2019 noted four instances of iris-stent touch.9 In our case, removal of the device led to improvement, but not resolution, of symptomatic iritis.
In conclusion, device malposition causing symptomatic iritis is an uncommon complication of Schlemm’s canal microstent placement. Removal of the implant may be necessary to control intraocular inflammation. However, this may not always lead to the resolution of inflammation, and longer-term steroid use coupled with glaucoma filtering surgery may be required.
1. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 2015;122:7:1283-1293.
2. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study. Ophthalmology 2019;126:1:29-37.
3. Ahmed IIK, De Francesco T, Rhee D, et al. Long-term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology 2022;129:7:742-751.
4. Ondrejka S, Körber N. 360° ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol 2019;13:1235-1246.
5. Dickinson A, Leidy L, Nusair O, et al. Short-term outcomes of Hydrus microstent with and without additional canaloplasty during cataract surgery. J Glaucoma 2023;32:9:769-776.
6. Heersink M, Dovich JA. Ab interno canaloplasty combined with trabecular bypass stenting in eyes with primary open-angle glaucoma. Clin Ophthalmol 2019;13:1533-1542.
7. Capitena Young CE, St Peter DM, Ertel MK, Pantcheva MB. Hydrus microstent malposition with uveitis-glaucoma-hyphema syndrome. Am J Ophthalmol Case Rep 2022;25:101405.
8. Kaplan TM, Sit AJ. A case of uveitis-glaucoma-hyphema syndrome related to a Hydrus microstent. J Glaucoma 2024;33:1:51-54.
9. Duong AT, Yuan M, Koenig LR, Rodriguez GH, Van Tassel SH. Adverse events associated with microinvasive glaucoma surgery reported to the Food and Drug Administration. Ophthalmol Glaucoma 2021;4:4:433-435.



