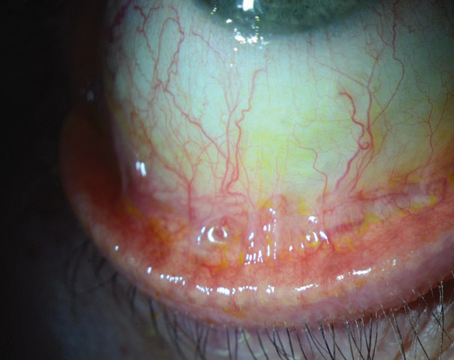
Dry eye is one of the most common problems seen in any eye-care office, but diagnosing it—especially in the early stages—has always been difficult. If you count diagnostic questionnaires, more than 60 different tools are now available for this purpose in the clinic and laboratory. Nevertheless, even the three most often used in the clinic (Schirmer strips, ocular surface staining and tear breakup time) have limitations and drawbacks.
The most promising option, in the eyes of many experts, is measuring osmolarity: the concentration of solutes in the tear film. Unlike other dry-eye measures, osmolarity is a biomarker, providing quantifiable physiological data of the sort most other branches of medicine rely on routinely, but which has been rare in eye care. Furthermore, many studies over the years have confirmed the value of osmolarity. [See "Osmolarity: The Most Accurate Test?" below.] That accumulated data was part of the reason that in April of this year the International Dry Eye Workshop issued a new report amending the definition of dry eye to include osmolarity.1
The real problem with using osmolarity as a way to diagnose dry eye has always been the difficulty of measuring it. Until recently, this required time-consuming tests that could only be done in a laboratory by an experienced doctor or technician. Now, that's changing. Two new instruments have been developed to make testing osmolarity much easier; one has just become available in the
Here, spokesmen for the two companies talk about the new instruments and how they hope to change the diagnosis and monitoring of dry eye for the better.
Point-of-Care Testing
One company, OcuSense, located in
"Most dry-eye tests have looked at physical endpoints or parameters that are late-stage signs of dry-eye disease," he says. "These don't necessarily correlate well with early-stage disease pathology. Also, the most commonly used tests—the Schirmer strip, ocular surface staining and breakup time—are subjective and qualitative, have poor specificity and take up valuable chair time because physicians usually perform the tests themselves. In contrast, our TearLab osmolarity test establishes an objective, physiological endpoint that has tremendously high specificity and provides a way to quantitatively grade disease severity. Moreover, TearLab has been designed to enable technicians to rapidly and accurately perform the test."
Mr. Donsky says that difficulties associated with collecting and evaluating tear samples accurately and repeatably in an office setting have presented a significant hurdle. "Some of the technical challenges include sample dilution caused by reflex tearing, sample evaporation and obtaining a precise measurement with less than 50 nanoliters of tear film," he says. "We believe our system has solved all of these problems in a way that will make its use practical in any physician's office."
Microfluidics: A Lab on a Chip
Mr. Donsky formed OcuSense in 2003 with biomedical engineer Benjamin Sullivan, the son of David A. Sullivan, PhD (known for his work with endocrinology and dry eye at Schepen's Eye Research Institute in
"It's very difficult to collect a large volume of tear film from a patient without inducing reflex tearing or overstimulating the lacrimal gland," Mr. Donsky explains. "Reflex tearing leads to more diluted tears, shifting the concentration of the biomarker in the fluid you're testing and creating a lot of variability. Also, most dry-eye patients have a reduced supply of tears, so the only way to collect a larger sample in many cases may be to generate reflex tearing, with its attendant problems.
"As a result, we had to find a way to extract the information needed from a much smaller sample, in the nanoliter range," he continues. "The smaller sample would let us get in and out of the eye so quickly that we could avoid generating reflex tears, and it would make it possible to test patients who have a very small volume of tears. We settled on the lab-on-a-chip solution because it's a volume-independent approach. Our system can accurately determine the osmolarity of a 40-nanoliter sample; that's about the volume of ink you get if you tap a pen very lightly on a piece of paper, or about 1/300th of a drop of water."
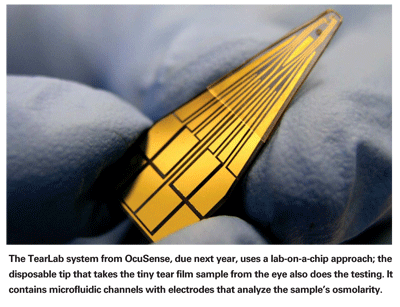
Mr. Donsky explains that the chip in question contains tiny microchannels that the fluid is pumped into. [See image, top.] A proprietary impedance spectroscopy technique is then used to determine the osmolarity. "A similar technique is used in blood glucose monitors," he notes. "It's well accepted by the FDA, in contrast to an exotic biosensing or biodetection system that would have a hard time getting 510K clearance and CLIA (Clinical Laboratory Improvement Amendments) waiver. And it's this type of testing that makes it possible to work with such a tiny tear sample."
Avoiding Evaporative Loss
A second key problem was preventing evaporation from altering the osmolarity of the sample—a more serious concern when the sample is so tiny. "If the sample is exposed to air, evaporative loss is instantaneous," Mr. Donsky notes. "Using a standard microcapillary to capture a sample would require interfacing with the eye, then interfacing the capillary to the chip, providing more opportunity for evaporation to occur. Also, a standard microcapillary can break, and it's difficult to be sure you have enough fluid. All of this adds to work-flow complexity and reduces the probability of obtaining a CLIA waiver from the FDA.
"So, we had to innovate a whole new way to collect tears," he continues. "The solution we settled on was to turn the testing chip itself into the tear collection interface to the patient's eye. This required developing several new technologies to ensure that the front end of the chip could wick up tears as efficiently as a glass capillary.
"Once collected, the tears are transported down the microfluidic channel to electrodes that perform the measurement on the chip within seconds," he says. "The use of the microfluidics greatly mitigates the risk of evaporation."
Mr. Donsky adds that the company also holds several patents on methods for eliminating noise and corruptive dynamic signals within nanoliter tear osmolarity testing. "The dynamic signal processing within our TearLab system is sophisticated enough to account for any real time evaporation within the sample," he says.
Conducting the Test
The single-use chips are designed to snap into a handheld "pen" that's reusable; the pen contains software that completes the measurement. "The pen was designed with a lot of input from both doctors and technicians in focus groups," says Mr. Donsky. "It's user-friendly and easy to hold whether you're right- or left-handed, regardless of your hand size. And it has sight-lines that allow you to look down into the tear lake from either side.
"Once the technician docks the pen to the main body of the instrument," he continues, "the instrument converts the electrical signal created on the pen into a reading of osmolarity. The base instrument is small; it has a footprint about the size of a telephone and easily fits on a shelf of an examination lane in a doctor's office. [See picture, below.] It comes with two pens and docking spaces so you can test one eye and then the other without waiting for the first test to be completed.
"Assuming the technician docks the pen immediately after taking the sample, the entire process only takes about 30 seconds to produce a reading," he notes. "And it's easy for a technician to use, which we demonstrated during a clinical study in
The test was also designed to avoid being categorized as high or moderate complexity under CLIA guidelines. "Most doctors' offices are not set up to handle the requirements that accompany tests rated as moderate or high complexity," notes Mr. Donsky. "Those require special training for the technicians, audits by the FDA and paperwork supporting every test. In fact, there are only about 400 ophthalmologists in the 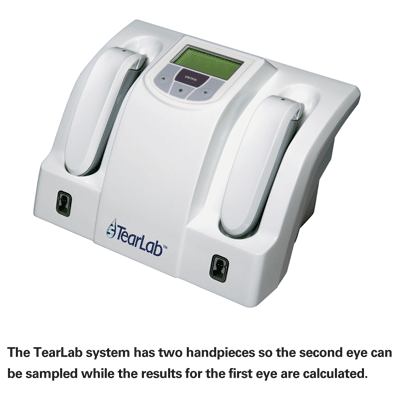
The TearLab isn't available yet, but should be widely available in Europe next year, with limited availability in the
In terms of what the company plans to do next, Mr. Donsky notes that the electrochemical testing technology used in TearLab isn't limited to quantifying osmolarity. "We've already licensed related technologies that should allow us to test for other biomarkers, such as specific proteins—not an easy task when dealing with such small volumes of fluid. And it should be possible to measure multiple biomarkers on a single chip, an innovation that could enable physicians to correlate key clinical endpoints."
The Tear Osmometer
An instrument based on a different method of measuring osmolarity has been developed by Advanced Instruments of Norwood, Mass. Advanced Instruments has been manufacturing laboratory and medical instrumentation for many years, and is expert at creating instruments that measure freezing point depression—i.e., how much the freezing point of a liquid has been lowered by the presence of solutes, which indicates the liquid's osmolarity. (Technically, OcuSense's TearLab measures osmolarity, or concentration of solutes per unit of volume; Advanced Instruments' Tear Osmometer measures osmolality, or concentration of solutes per unit of weight.) The Tear Osmometer recently became widely available for the first time, making it the first tear quality assessment instrument to reach the marketplace.
"Although our company has been around for more than 50 years, this is the first instrument we've developed for the ophthalmic industry," says Chris Corbett, senior project engineer at Advanced. "We developed our first prototype 10 years ago, and we've had a lot of input from experts in the dry-eye field.
"Our company creates laboratory diagnostic instrumentation that makes precision freezing point measurements, whether the fluid being tested is blood, urine, milk or other liquids," he explains. "A mathematical conversion of the freezing point gives you the ionic concentration, or osmolality. The challenge in this case was to make the precision measurement on a nanoliter-sized sample, which can be difficult due to evaporation, sample handling and potential contamination."
Determining Osmolality
To use the Tear Osmometer, the doctor takes a small tear sample using a plastic capillary (avoiding potential breakage concerns that would apply to glass); the sample is then transferred to a special tip that is inserted into the main instrument. The instrument freezes the sample and measures the freezing point using a dedicated PC and custom software. The PC also shows the sample visually, magnified 180 times, so you can watch the liquid freeze and thaw. [See image, below.]
Mr. Corbett sees advantages to using freezing point depression to determine osmolality, rather than an alternative process such as electrochemical analysis. "Freezing point depression is a colligative property that follows basic laws of physics," he explains. "It depends solely on the concentration of dissolved substances —not on their nature. Unlike some other approaches to measuring biochemical characteristics, this is extremely precise and not subject to interpolation. That's important, because when you're measuring tear osmolality, the difference between being normal, mild, moderate or severe is so small that you have to have incredible precision in your measurements."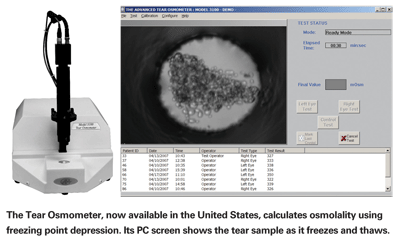
Mr. Corbett acknowledges that the Tear Osmometer requires a somewhat larger tear sample than the technology being developed by OcuSense, but doesn't see this as a major drawback. "We polled a number of MDs on what would be a reasonable volume to collect from a dry-eye patient," he says. "It's my understanding that a normal patient can provide about 10 µl in sample volume. Our instrument requires 0.5 µl, or 500 nanoliters, of sample fluid; if we test much less than that we begin to lose accuracy and repeatability. Admittedly, some patients with severe dry eye might not be able to produce that volume of basal tears, but most doctors have told us that our limit will still allow us to evaluate mild, moderate and some cases of severe disease. And at the outset, the most severe cases may not be the ones researchers are anxious to test."
Mr. Corbett says that once the sample is in the capillary it's not exposed to any air flow. "The opening is so small that little evaporation can take place," he explains. "The sample is then transferred to a fabricated tip that goes into the main instrument for analysis. The transfer process could be subject to some evaporation if it's not done carefully and quickly, but the tip itself is designed to minimize evaporation as long as you don't let it sit around for several minutes before placing it into the main instrument and starting the test. The bottom line is this: As long as the test is done in one smooth sequence, our results show no impact from evaporation."
As for the potential problem of generating reflex tearing when taking the sample, Mr. Corbett says the company relies on the expertise of the ophthalmologist to collect the sample from the eye without causing tearing. "Our expertise is manufacturing the instrumentation," he notes. "Most ophthalmologists are experienced at collecting tears, and the capillary device that we provide is identical to the glass capillaries doctors use to collect tear samples for other types of analysis. From what I've seen, as long as the tear volume exists, if you use proper technique and come in from the side you can collect good samples without inducing reflex tearing."
A Tool with Potential
Mr. Corbett says the current 
The company's focus, for now at least, is on reaching research ophthalmologists. "That's primarily driven by the current configuration of our instrument," says Mr. Corbett. "It has some limitations that might make it more challenging to use in a clinical setting, such as test time and the lack of an established reimbursement or CPT code. [For more on this, see "Getting Reimbursed," above.] Our feedback has mostly been that it makes sense to begin by targeting the research ophthalmologist."
On the other hand, the company hasn't ruled anything out. "This is the only instrument commercially available right now that gives a numeric value associated with osmolality," Mr. Corbett says. "That makes it possible to categorize a patient as normal, mild, moderate or severe dry eye; to see if there are correlations to various illnesses or diseases; and to monitor the effectiveness of drug therapies. Our plan is to see how well the instrument is received and how large the market really is.
"Given the tiny size of the sample, it's remarkable to achieve this level of accuracy and repeatability," he adds. "We're very excited to be making this commercially available, with all that it means for the diagnosis, monitoring and treatment of dry-eye disease."
Only the Beginning
Now that osmolarity has been amended into the definition of dry eye in the DEWS report, Mr. Donsky says he hopes to see osmolarity become an FDA-accepted endpoint for therapeutic development. "We're just at the beginning of osmolarity testing," he notes. "We need objective endpoints for clinical trials. There are so many potential drug compounds in the development pipeline that could benefit from an endpoint like osmolarity that can further substantiate therapeutic efficacy to the FDA. We're working with several pharmaceutical companies to establish osmolarity as a primary or secondary endpoint in their clinical protocols. We hope that this will expedite regulatory approvals."
In any case, both Mr. Corbett and Mr. Donsky see biomarker analysis as an important advance in eye care. "In other disease categories—cardiovascular, oncology, infectious disease, autoimmunity—doctors depend on the analysis of biomarkers for making decisions," notes Mr. Donsky. "In the eye-care industry, we haven't had this capability. Having it will allow doctors to differentially diagnose disease with more sensitivity and specificity, stratify the patient population by etiology and physiology, and enable a quantitative tracking of patient outcomes to selected treatment regimes. It's an important step forward."
1. Methodologies to Diagnose and Monitor Dry Eye Disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop. The Ocular Surface 2007; 5:2:108-152.
2. Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A.
Tear film osmolarity: Determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:10:4309-15.