|
Mark B. Abelson, MD, Aron Shapiro, and Ingrid Lapsa |
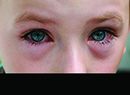
Bacterial conjunctivitis is the infectious bane of daycares, schools, and institutionalized living communities, dreaded for its uncomfortable symptoms and ability to spread.
As evidence of its infectious nature, one need look no further than the winter 2002 outbreak at Dartmouth College. There, 13.8 percent of students on campus were diagnosed with bacterial conjunctivitis, including 22 percent of the first-year student class. The attack rate caused such concern that the state Department of Health enlisted the aid of the Centers for Disease Control and Prevention to investigate and manage the crisis. The responsible pathogen was identified as Streptococcus pneumoniae. Factors associated with infection included close contact with an infected student, wearing contact lenses, membership on a sports team and attending parties.1
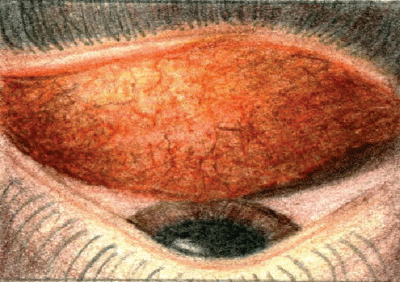 |
| Papillae will be present in eyes with conjunctivitis of bacterial origin. All illustrations: Ingrid Lapsa |
A campaign promoting hand washing and early treatment of symptoms—while warning against sharing drinking glasses, towels and utensils—was launched. Between heightened awareness, prompt treatment, and perhaps departures for spring break, the outbreak was quashed.
Primary-care physicians see infectious conjunctivitis more than any other ocular disorder, and the incidence is highest in children. Treating bacterial conjunctivitis with an agent offering broad-spectrum coverage and a rapid onset of action is the key to preventing possible sequelae, including a corneal ulcer resulting from a break in the ocular surface in the presence of pathogenic bacteria. Quick treatment is imperative for reducing the infection's opportunity to spread.
The Infection
Conjunctivitis refers to a number of conditions. These may be infectious or non-infectious, and acute, hyper-acute or chronic. Ocular redness is apparent in nearly all cases, though other aspects of presentation vary. Care must be taken to differentiate bacterial infections from viral diseases and allergic conditions.
The three most common causative pathogens in acute bacterial conjunctivitis are Haemophilus influenzae, Streptococcus pneumoniae, and Staphylococcus aureus. Children are most commonly afflicted with S. pneumoniae and H. influenzae, the latter accounting for the majority of cases.2 S. aureus infections are more frequent among adults. In our experience with acute bacterial infections, warm and humid climates, crowded living conditions and poor personal hygiene have emerged as additional risk factors.
Symptoms are similar regardless of the pathogen. The definitive symptom is copious mucopurulent discharge, such that patients' eyelids often are pasted together upon waking. Injection of the bulbar and palpebral conjunctiva is evident, earning the condition the colloquial name of "pink-eye." There may be papillae, chemosis and mild lid edema, and patients may report ocular burning, irritation and tearing. Most often, symptoms begin in one eye and spread to the other. Significant or progressive lid edema may herald orbital cellulitis.
Acute bacterial conjunctivitis is usually self-limited and doesn't generally pose any serious threat to vision. Hyper-acute infections are much more serious, sight-threatening conditions. The pathogen most often responsible is Neisseria gonorrhoeae, followed by the far less common Neisseria meningitidis.
N. gonorrhoeae infections typically appear in newborn infants and sexually active young adults.3 Neonates contract the disease by passing through an infected birth canal. Adults most often introduce the organism to their ocular tissue with their hands, after having contacted infected genitalia. Rapidly progressive symptoms develop within a few days. These include excessive yellow-green purulent discharge, conjunctival injection and chemosis, lid swelling and tender preauricular adenopathy. Without treatment, corneal involvement is unavoidable. Infiltrates, ulceration and perforation can lead to permanent loss of vision.
The organism Chlamydia trachomatis is responsible for other potentially sight-threatening infections. There are two distinct clinical manifestations of ocular C. trachomatis: trachoma and the milder inclusion conjunctivitis.
Trachoma primarily affects rural societies in developing countries.4 Unsanitary living conditions and limited access to clean water are also risk factors. It's a chronic condition, usually first contracted during childhood. Repeated re-infection leads to conjunctival scarring, corneal involvement and eventual blindness.5 Transmission is believed to occur through direct contact with hands or fabric that have been contaminated with ocular or nasal secretions. In some regions, eye-seeking flies are also thought to spread the organism.
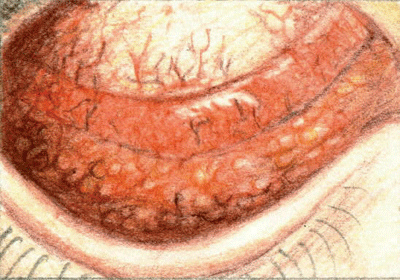 |
| Follicles are the hallmark sign of viral conjunctivitis. |
While trachoma is rare in the United States, communities marked by poverty, crowded living conditions, and/or poor hygiene remain at higher risk for it. Incidence may also be higher among immigrants from regions where trachoma is prevalent.
Venereal transmission is typical of inclusion conjunctivitis. Infants and sexually active adults are again most frequently affected. Infants are infected during birth, and begin exhibiting bilateral mucopurulent discharge, lid swelling and chemosis within five to 14 days. In adults, the infection often presents as sub-acute or chronic, and may be limited to only one eye. Symptoms are the same as with infants, but also include the emergence of follicles in the palpebral conjunctiva and papillae in the tarsal conjunctiva. Foreign-body sensation and preauricular adenopathy are common. In some cases, corneal damage may result from scarring and neovascularization.
Diagnosis
When it comes to diagnosis, the importance of a thorough medical history cannot be overstated. In addition to aiding the identification of a particular sub-type of bacterial conjunctivitis, a detailed account of a patient's history is important. The symptom profiles of infectious and non-infectious conjunctivitis overlap, especially with regard to erythema. Characteristic signs and symptoms differ.
One simple rule is that acute bacterial conjunctivitis presents as papillae, viral conjunctivitis as follicular, and chlamydial conjunctivitis as both. Viral conjunctivitis also displays watery ocular discharge that doesn't mat the lids together, and a palpable pretragal or preauricular node is usually present.
Bacterial keratitis will usually be unilateral, with severe redness, sharp pain, photophobia and corneal edema. Though mucopurulent discharge is often present, it will be thick and ropy rather than sticky as in conjunctivitis.
Two other possible causes of conjunctival redness include dry eye and allergic conjunctivitis. As with viral conjunctivitis, purulent discharge is absent in dry eye. Symptoms include ocular burning, stinging and fatigue. Redness may appear as interpalpebral "banding" rather than even distribution across the surface of the eye.
In allergic conjunctivitis, redness often appears with pronounced veins. The key symptom is itching. A patient's description of when symptoms arise or worsen (e.g., during a particular season) may also implicate allergy.
A slit-lamp exam can reveal a great deal. Cultures are not usually warranted in cases of mild acute bacterial conjunctivitis, but severe, recurring or treatment-resistant cases may suggest a more serious problem. If a gonococcal or chlamydial infection is suspected, a culture should be obtained to either rule out the condition or begin treatment. Cultures should be performed with a dry, synthetic swab, such as Dacron or rayon, and wooden shafts should be avoided. An effort should be made to collect the epithelial cells of the palpebral conjunctiva, and care should be taken not to touch the lashes, lids or any mucous discharge. A discussion of culture techniques can be found in our September, 2002 column.
Both blood agar and chocolate agar should be used as media during incubation. Chlamydia is identified through inoculation and incubation into a cell culture.
|
Treatment
As acute bacterial conjunctivitis is usually self-limited, it may resolve without treatment. Chancing this is a poor idea for a number of reasons, however.
For one, the disease is highly contagious. Failing to treat an infectious condition increases the risk of exposure to others. In addition, symptoms and lost days of work compromise a patient's quality of life. Many schools require children with the infection to be kept at home until they have been on antibiotics for at least 24 hours.
Furthermore, there remains the possibility the condition will not resolve without intervention, in which case the patient risks complications such as corneal infiltrates and even ulceration. In the worse case, scarring from a corneal ulcer, endophthalmitis, perforation or loss of an eye may occur if there is a break in the ocular surface. There also exists the risk of extra-orbital disease: Untreated H. influenzae type B infections can lead to systemic disease.6
When selecting an antibiotic to treat an acute condition, broad-spectrum topical medications offer multiple advantages. As cultures are rarely taken in acute cases, a wide-coverage agent usually ensures rapid microbial eradication. Ointments offer the benefit of prolonged contact with the ocular surface, and an adjunct soothing effect. Eye drops are generally better tolerated by children and don't interfere with vision, however.
Fluoroquinolones provide broad-spectrum coverage and boast low toxicity, rapid onset of action and better ocular penetration than any other topical antibiotic.7 Bacterial resistance has repeatedly demanded the development of new formulations, each generation aspiring to thwart the resistant strains. S. aureus is particularly adept at developing resistance.
The fourth-generation fluoroquinolones introduced in 2003 include gatifloxacin (Zymar, Allergan) and moxifloxacin (Vigamox, Alcon), both of which offer enhanced activity against gram-positive and gram-negative aerobic and anaerobic organisms. Interestingly, moxifloxacin has shown seven times the conjunctival concentration of gatifloxacin 20 minutes following instillation.8
Although moxifloxacin is at a slightly higher concentration than gatifloxacin, 0.5% compared with 0.3%, respectively, a study sponsored by Alcon has found that the higher tissue concentrations may be due to moxifloxacin's biphasic (both lipid and aqueous) solubility. (Rusinko A, et al. IOVS 2004;45:ARVO E-Abstract 4907) Further, moxifloxacin's package insert recommends t.i.d. dosing for seven days, while gatifloxacin's insert states dosing q2h for days one and two and q.i.d. for days three to seven. A reduced dosing regimen may result in greater patient compliance. A rapid, greater penetration may lead to accelerated bacterial kill.
Even as fluoroquinolones remain the top option, S. aureus and its peers have shown no signs of yielding for good: Studies suggest mutations can be specifically influenced by the particular drug to which they are exposed.9-11
Bacterial mutations are not the sole factor contributing to resistance. Misdiagnosis can result in a patient's overexposure to antibiotics, such as when a virus is treated with an antibiotic. One study found that while more than 80 percent of conjunctivitis cases are treated with antibiotics, only 32 percent of the cases tested were actually bacterial in origin. In a more recent study that we conducted, enhanced culture techniques and controlled site management resulted in a 46-percent culture-positive rate.13
Dosing adherence is another factor, and it can be difficult to achieve, particularly among pediatric patients. The fluoroquinolones can require as many as eight doses per day at the beginning of the treatment period, decreasing to q.i.d. for the remainder of the course (See box, p. 80). Conscientious dosing may be easier to achieve while symptoms are at their worst, but as they begin to subside, adherence often begins to slip as well. This gives hardy strains a window to renew their activity and develop resistances. It's imperative that patients complete the entire course of treatment.
Treating a Neisseria or Chlamydia infection can present more immediate challenges. The proper course of therapy should include a systemic antibiotic, a topical antibiotic and regular saline irrigation. For adult inclusion conjunctivitis, azithromycin (Zithromax, Pfizer) is a favorite. It is packed in a single 1- or 2-g dose, and patients then need only use one dosage schedule and maintain saline irrigation.
Ceftriaxone is preferred for gonococcal conditions, administered in a single 125-mg injection. Adults and their sexual partners should also undergo further testing for venereal diseases, as ocular involvement is often secondary to genital infection.
Infants should be hospitalized during treatment and carefully monitored for disseminated infection. To prevent spreading the disease to other infants, they should be kept isolated from the rest of the nursery. As with adults, a single intravenous or intramuscular dose (25-50 mg/kg for infants, not to exceed 125 mg) of ceftriaxone is recommended against Neisseria species. Against chlamydial conditions, oral erythromycin (12.5 mg/kg q.i.d. for 14 days) is recommended. Coupled with topical antibiotic therapy and regular saline irrigation, the prognosis for patients is usually very good.
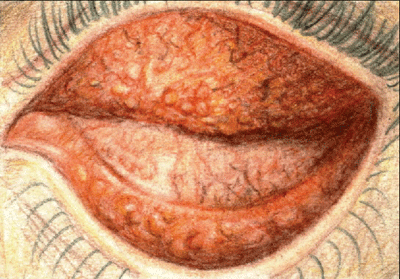 |
| Chlamydial conjunctivitis presents with both follicles and papillae. |
Other Considerations
Most patients enter the health-care system through the pharmacy shelf or family doctor. Many, especially pediatric patients, have never set foot inside an ophthalmologist's office. When a condition is believed to be acute and uncomplicated, a nurse practitioner may simply call in a prescription and the patient will never have an office visit. There is no harm in this strategy as long as the prescription is appropriate to the condition. In more critical or hyper-acute cases however, the delay of systemic treatment does the patient a disservice.
It is likely that a patient will be referred if symptoms do not clear as expected. Sometimes a detail undisclosed in a patient's medical history is all that stands between his current condition and a return to health. Sexual abuse can cause the rare pediatric case of hyper-acute conjunctivitis. No antibiotic will relieve the ocular redness of an allergic patient with a house full of cats. Poor hygiene can reintroduce bacteria daily as a child rests his head on a contaminated pillow.
Talking to pediatricians and referring physicians is an effective way to minimize misdiagnosis and over-prescription, and to have referrals issued sooner than later. As seen at Dartmouth, prompt care coupled with a sound hygienic regimen can make even college parties safe again.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Mr. Shapiro is the director of anti-infectives and Ms. Lapsa is a clinical research associate in allergy at ORA Clinical Research and Development in North Andover.
1. Gigliotti F, Williams WT, Hayden FG, et al. Etiology of acute conjunctivitis in children. J Pediatr 1981;98:4:531-536.
2. Vichyarond P, Brown Q, Jackson D. Acute bacterial conjunctivitis. Clin Pediatr 1986;25:10:506-509.
3. Morrow GL, Abbot RL. Conjunctivitis. Am Fam Phys 1998;57:735-746.
4. Courtright P, West SK. Contribution of sex-linked biology and gender roles to disparities with trachoma. Emerg Infect Dis 2004;10:11:2012-6.
5. Johnson GJ. The environment and the eye. Eye 2004;18:12:1235-50.
6. Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol 1991:112:29(S).
7. Mah FS. Fourth-generation fluoroquinolones: new topical agents in the war on ocular bacterial infections. Curr Opin Ophthalmol 2004;15:316-320.
8 . Wagner RS, Abelson MB, Shapiro A, et al. Evaluation of moxifloxacin, ciprofloxacin, gatifloxacin, ofloxacin, and levofloxacin concentration in human conjunctival tissue. Arch Ophthalmol 2005 Sep;123:9:1282-1283.
9. Griggs DJ, Marona H, Piddock JV. Selection of moxifloxacin-resistant Staphylococcus aureus compared with five other fluoroquinolones. J Antimicrob Chemother 2003;51:1403-1407.
10. Pumbwe L, Randall LP, Woodward MJ, Piddock LJV. Evidence for multiple-antibiotic resistance in campylobacter jejuni not mediated by CmeB or CmeF. Antimicrob Agents Chemother 2005;49:4:1289-1293.
11. Ricci V, Peterson ML, Rotschafer JC, et al. Role of topisomerase mutations and efflux in fluoroquinolone resistance of bacteroides fragilis clinical isolates and laboratory mutants. Antimicrob Agents Chemother 2004;48:4:13344-1346.
12. Rietveld RP. Predicting bacterial cause in infectious conjunctivitis: Cohort study on informativeness of combinations of signs and symptoms. Brit Med J 2004;24;329:7459:206-210.
13. Data on File, Ophthalmic Research Associates.