In this month’s column we’ll take a look at additional outcomes driven by environmental influences. With an estimated 5 million dry-eye sufferers1 and only one approved drug for its treatment (Restasis, Allergan), the placebo effect and other confounders play significant contributing roles in the failure of many registrational studies. A fully developed understanding of the impact of the factors pressing on the dry-eye population can potentially help to advance efforts to find relief.
 Environmental Challenges
Environmental Challenges
Dry-eye symptoms can be triggered by a number of causative factors including, but not limited to, prolonged visual tasking, ocular surgery, aging, oral antihistamines and systemic medications,2,3 and are especially exacerbated by a variety of environmental stresses. Visual tasks such as reading or using a computer for an extended period of time can lead to infrequent blinking, which causes the tear film to break up more quickly, generating an unprotected ocular surface.4,5 For most patients, these visual tasks are unavoidable, as they are part of their daily lives.
Dry-eye patients must also be aware of their surrounding environment, as it greatly impacts their disease and, ultimately, their quality of life. In addition to the aforementioned factors, seasonal and geographical challenges remain a crucial consideration in managing dry eye.6
Those patients who live in the Northeast region of the United States undergo significant changes in the environment with each seasonal change. During the winter months, dry eye can be especially challenging as the air is drier and the humidity is decreased both outdoors and indoors.7 A step outside gives way to problematic weather, such as cold, brisk air and wind but even inside, stresses like hot air vents, fireplaces, wood stoves and other fumes can potentially worsen dry eye. Car vents are also a source of hot, dry air that can exacerbate symptoms. Additionally, the low winter temperatures force individuals to spend much more time in proximity with one another indoors, where everyone will be more likely to catch or give colds. This may lead to the use of decongestants and other medications that can dry the ocular surface; avoiding such drugs as much as possible may help prevent further discomfort for dry-eye patients.
| The Classic Dry-eye Patient |
| The typical dry-eye patient is a 50-year old, post-menopausal woman who enjoys the summer months greatly in her New England home. She spends a majority of her time outside, as the air is humid and it rains quite frequently. Unfortunately, with the change in season, she begins to spend her days inside reading and on the computer; consequently, she begins to feel burning, stinging and grittiness in her eyes, indicating that her dry-eye symptoms have increased. Making matters worse, her house is heated with a wood stove and her time spent outside the house is in the car, with the hot air circulating. Ultimately, she experiences increased signs and symptoms (and potentially keratitis) during the winter months, and, as the season draws to an end, her dry-eye signs and symptoms slow as well. |
Finally, since these environmental challenges have a significant impact on dry-eye patients, it only makes sense that they also affect dry-eye clinical studies.
Placebos in Clinical Trials
In order to determine the efficacy of a drug, U.S. registrational drug studies are regulated by federal law and required to compare drug effects on signs and symptoms in a treatment arm to a placebo group. In some instances, between-group comparison of results can be distorted by what is known as the placebo effect, which is a measureable, observable or perceived improvement not attributable to an active treatment. One phenomenon that can give way to misleading results, especially in dry-eye studies, is the fact that the placebo used is likely to produce a positive therapeutic effect because of its inherent lubricant therapy.
It’s common practice to use the vehicle of an investigational therapy as a control. It should be noted, however, that all vehicles are not created equal and, based on its physical characteristics, the vehicle therapy may have an unexpected effect. For example, a more viscous vehicle, such as a suspension, can be more effective in reducing signs and symptoms of dry eye than a less viscous vehicle. Dry-eye studies are unique in that the placebo being used is actually a therapy in itself and acts as a protectant. It’s believed that unpreserved normal saline may be beneficial in flushing out debris, diluting substances trapped in the tear film and increasing tear clearance.10
 |
Regression toward the mean is based on the notion that patients are entered into a clinical study when their symptoms are at their worse (i.e., to meet inclusion criteria) which means they are much more likely to improve over the course of the study.
Understanding that these factors are influential is important when conducting dry-eye clinical trials and choosing the right patients. Dry-eye patients experience a worsening of signs and symptoms during the heightened dry-eye seasons (usually the winter months in certain parts of the country). When these patients enter the dry-eye season, they are much more apt to feel the effects of the environmental and seasonal changes. The Controlled Adverse Environment (CAE) model, an environmental chamber that exacerbates the signs and symptoms of dry eye by regulating humidity, temperature, airflow, lighting conditions and visual tasking,8 has been used as a screening tool to identify and enroll patients who are more susceptible to environmental insults. To help minimize the previously discussed influences, it’s crucial to enroll patients during and within the dry-eye season. Furthermore, utilizing a tool like the CAE model to help select patients who are susceptible to these seasonal effects can notably minimize and/or augment treatment response.11 (Ousler GW III, et al. IOVS 2010;51: ARVO E-Abstract 3362) Researchers from Ora also demonstrated how the enhanced Controlled Adverse Environment model can increase within-subject reliability in a poster presented at the 2010 meeting of the Tear Film and Ocular Surface Society.
All of the aforementioned occurrences (the placebo effect, the lubricating therapy of placebo in dry-eye studies, the Hawthorne effect and regression toward the mean) affect the improvement of a placebo group in a clinical study of a drug. These four factors are working together in one direction to make the placebo appear to act more like a drug, while at the same time seasonality and environmental factors are pulling in the opposite direction, and can contribute to the deterioration of the placebo’s effects on the signs and symptoms of the disease as the winter season progresses. The “red triangle” effect is an example of the environmental influences on dry-eye patients (See Figure 1).
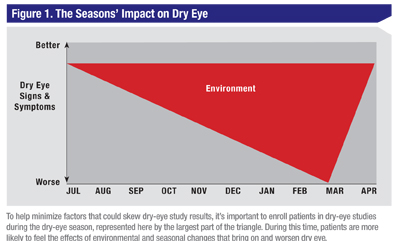 Clinical trials conducted within the “red triangle” season may demonstrate either an amelioration or prevention of the signs and symptoms of dry eye such as keratitis, conjunctival staining, conjunctival redness and ocular discomfort. A therapy that is prophylactic in the exacerbation of keratitis during this heightened season would be beneficial, since the worsening of ocular surface damage can influence vision and patient comfort. A dry-eye therapy such as this would theoretically create an endless summer, extending the ideal season for dry-eye patients’ ocular surfaces.
Clinical trials conducted within the “red triangle” season may demonstrate either an amelioration or prevention of the signs and symptoms of dry eye such as keratitis, conjunctival staining, conjunctival redness and ocular discomfort. A therapy that is prophylactic in the exacerbation of keratitis during this heightened season would be beneficial, since the worsening of ocular surface damage can influence vision and patient comfort. A dry-eye therapy such as this would theoretically create an endless summer, extending the ideal season for dry-eye patients’ ocular surfaces.
Impact of Environment
When conducting a clinical trial, keep in mind the various challenges that dry-eye subjects face.
Classically, environmental studies of dry-eye medications have been conducted across many seasons, sometimes continuing to go on for multiple years. It’s not surprising that many of these studies have failed, perhaps not due to the drug’s efficacy itself, but because of the study design, because as patients enter and leave the different dry-eye seasons of the year, the unpredictability of critical environmental factors make it difficult for the clinical investigators to measure the signal of a drug effect. Dry-eye patients constantly find themselves walking on a high-wire, and as such, there is very little room for disturbance in their condition, be it from daily routines or their environment. Any small change in habit, such as staying up an hour later or using the computer longer, can cause dry-eye patients to experience heightened signs and symptoms. Therefore, medications that can help to prevent the worsening of dry eye can serve to keep dry-eye symptoms in check. Additionally, using the established technologies to select the proper patient can yield a better controlled dry-eye study and a potentially greater treatment effect.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Mr. Ousler is director of the dry-eye department and Ms. Lafond is a medical writer at Ora Inc., in Andover.
1. International Dry Eye Workshop. The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye Workshop. Ocul Surf 2007;5:2:93-107.
2. Welch D, Ousler GW III, Nally LA, Abelson MB, Wilcox KA. Ocular drying associated with oral antihistamines (loratadine) in the normal population: An evaluation of exaggerated dose effect. Adv Exp Med Biol 2002;506(Pt B):1051-1055.
3. Ousler GW, Wilcox KA, Gupta G, Abelson MB. An evaluation of the ocular drying effects of two systemic antihistamines: Loratadine and cetirizine hydrochloride. Ann Allergy Asthma Immunol 2004;93:5:460-464.
4. Karson CN, Berman KF, Donnelly EF, Mendelson WB, Kleinman JE, Wyatt RJ. Speaking, thinking, and blinking. Psychiatry Res 1981;5:3:243-246.
5. Patel S, Henderson R, Bradley L, Galloway B, Hunter L. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci 1991;68:11:888-892.
6. Abelson MB, Ousler GW, 3rd, Nally LA, Emory TB. Dry eye syndromes: Diagnosis, clinical trials and pharmaceutical treatment-—‘Improving clinical trials.’ Adv Exp Med Biol 2002;506(Pt B):1079-1086.
7. Davis J, Ousler GW III, Langelier NA, Schindelar MR, Abelson R, Abelson MB. Seasonal Changes in Dry Eye Symptomatology. Invest Ophthalmol Vis Sci(Poster) 2006;47.
8. Ousler GW III, Gomes PJ, Welch D, Abelson MB. Methodologies for the study of ocular surface disease. The Ocular Surface 2005;3:143-154.
9. Uchiyama E, Aronowicz JD, Butovich IA, McCulley JP. Increased evaporative rates in laboratory testing conditions simulating airplane cabin relative humidity: An important factor for dry eye syndrome. Eye Contact Lens 2007;33:4:174-176.
10. Tananuvat N, Daniell M, Sullivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea 2001;20:8:802-806.
11. Patane AM, Cohen A, From S, Torkildsen G, Welch D, Ousler GW III. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) for the treatment of dry eye: Results of a randomized clinical trial. Clinical Ophthalmology; in press, 2011.