Ophthalmology textbooks will tell you that there are five or six types of ocular allergies: seasonal; perennial; atopic; vernal; and drug- or contact lens-induced. Perhaps at one time, these different conditions were all a single diagnosis in the same way that all cancers were once considered the same disease. Those times are long past, and so it is with ocular allergy. As new therapies are tested and refined, as basic scientific inquiry further elucidates cellular mechanisms and pathways, the distinctions between these different types of ocular allergy come into focus. What we’ve learned, as have many medical subspecialties, is that one size doesn’t fit all. This month, we will focus on one of these types of ocular allergy, vernal keratoconjunctivitis. While we examine the features of VKC we’ll see how it differs from other types of allergy, and discuss how these differences are being used to identify therapeutic strategies for each type.
Webster’s defines the adjective “vernal” as relating to, or occurring in the spring. A secondary definition is that it refers to something fresh, young or youthful. Both of these definitions are appropriate descriptors for VKC, a chronic allergic disorder that typically presents as a seasonal conjunctivitis in patients who average 5 to 10 years of age. It’s the chronic nature of the disease that makes VKC so potentially devastating to its sufferers. Without aggressive, long-term therapy, the disease can lead to corneal ulcers, keratoconus and permanent visual impairment.
Descriptions of VKC in the ophthalmic literature date back several hundred years.1 Despite the apparent allergic nature of the disease, the precise causes have remained obscure. Many prominent features of the disorder don’t fit the criteria established for ocular allergy in general. Foremost among these is the prevalence in prepubertal boys, the geographic restriction to equatorial regions and the tendency of the disease to recur and intensify in the absence of typical allergic triggers. There are uncertainties regarding an often-suggested association between atopy and VKC, as many of the features commonly observed in atopic patients are absent in those with VKC.2 For example, skin sensitization tests, radioallergosorbent tests and serum or tear IgE levels are equivocal in these patients, with some studies showing a correlation and others showing none. Patterns of cytokine production are also inconclusive; a recent analysis of patients with VKC demonstrated increases in both the allergy-associated IL-4 and the inflammatory cytokine interferon-γ.3 The picture that emerges is a disease with some allergic components in combination with a prominent chronic inflammatory response.
Another feature of VKC that reflects a complex etiology is its prevalence in boys. The disease is three times more common in boys than girls, and tends to resolve spontaneously as patients of both sexes progress through puberty. While there are other examples of ocular surface diseases in which hormones such as estrogen or testosterone are known to play a role (e.g., dry-eye disease), the mechanisms that underlie this effect are unclear. Similarly, the geographic distribution of VKC is well-established but not entirely understood. In the Middle East and in African countries, from the Mediterranean to as far south as central Africa, VKC accounts for approximately 5 percent of all ophthalmic patients.4 It is also common in Central America and in India. In contrast, surveys conducted in Europe and in the United States show an overall incidence that is in the range of 0.01 to 0.03 percent, or less than 1 percent of that observed in more equatorial regions.4
Recent epidemiological reports provide evidence of a genetic component to the disease. Studies of patients living in regions where the disease is rare (such as Europe) show that the majority of VKC sufferers are first- or second-generation immigrants from areas where the disease is endemic.1,4 Other studies have established that a reduction in tear diamine oxidase activity is also associated with VKC, at least in some patients.5 This enzyme deficiency results in significant increases in ocular histamine and thus could represent an early event in a chronic disease cascade. Such single-enzyme defects can often be the result of inheritable mutations, and so may provide a molecular basis for the geographically and genetically restricted nature of the disease. Additional studies on the association between diamine oxidase deficiency and VKC are needed to address these possibilities.
|
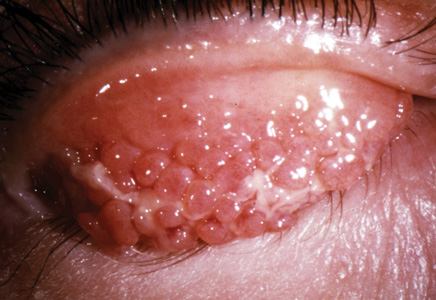
The initial presentation of VKC may not be significantly different from that of seasonal allergic conjunctivitis. Patients typically complain of bilateral ocular itching, pain and photophobia. They also may present with hyperemia, tearing and a stringy ocular discharge.6 As the disease progresses, however, several features that are classically diagnostic for VKC develop that distinguish it from either seasonal or perennial allergic disease. These include the characteristic “cobblestone” tarsal papillae and limbal gelatinous infiltrates. Most often patients present with either tarsal or limbal signs, but more severe cases with both features may also occur. Following initial presentation, signs and symptoms can wax and wane for years. Typically VKC displays a seasonality such that recurrences develop in spring, but often the severity and precise timing of disease symptomatology are sporadic.1,2,6
Differential diagnosis involves a number of factors.1,2,7 The geography of disease prevalence overlaps with the distribution of trachoma, so patients will often have these two eye diseases simultaneously. Of course, initial treatment should address this possibility. Patients with AKC can be distinguished from those with VKC in that atopic disease occurs later in life (after 30 years of age), typically occurs in combination with atopic dermatitis or eczema, and rarely exhibits tarsal or limbal features of VKC. Cytology, including corneal scraping or impression cytology, reveals prominent immune cell infiltration of the conjunctiva, and this trait may be maintained even during periods between outbreaks of symptomatic disease. Progressive growth of papillae results in expansion of the tarsal epithelium and reduction of the stromal layer. In the limbal form of the disease, aggregates of immune cells and fibroblasts form punctate limbal bodies called Tranta’s dots. Without treatment, chronic inflammation of the ocular surface can lead to formation of corneal shield ulcers, corneal neovascularization and possible keratoconus.
Targeting Chronic Inflammation
|
The introduction of aspirin as a therapy for VKC was a major advance, and it established the understanding of the central role inflammation plays in the etiology of the disease.8 Other non-steroidal anti-inflammatories such as ketorolac or nepafenac are sometimes used to provide relief for pain and inflammation, although they are relatively ineffective at treating the more severe forms of the disease. For example, they don’t have a significant effect on size or inflammation of tarsal papillae.9 In addition, aspirin-like compounds are associated with an increased risk of Reye’s syndrome, a particular concern in the young population of VKC patients. For these reasons corticosteroids are generally accepted as the anti-inflammatory of choice.6,7,9
Short courses of topical steroids are often necessary for moderate to severe outbreaks of VKC, especially those that feature tarsal or limbal inflammation. The course of required treatment can be several weeks, and severe cases often require multiple courses. Even with such regimens, resolution of papillae can take many months and thus often lags behind complete symptomatic relief. While continuous steroid therapy can hasten the healing, it also increases the likelihood of the side effects that limit all ocular steroid therapy, namely increases in intraocular pressure and risk of cataract. One recent study reported that steroid-induced cataracts may occur in as many as 10 percent of all AKC patients.4
The severity of the disease, in combination with risk factors associated with the use of either NSAIDs or corticosteroids in a pediatric population, has led to an increase in efforts to identify alternative therapeutic strategies for VKC.
Several recent clinical trials10-14 have examined the efficacy of anti-inflammatories such as cyclosporine or tacrolimus for treatment of VKC. Topical cyclosporine is a drug that is approved for ocular use and has an extensive track record for safety and a low risk of adverse events. It’s currently used in patients with aqueous-deficient dry-eye disease as a topical emulsion containing 0.05% cyclosporine.15
Trials of cyclosporine for VKC have employed a range of concentrations (0.05% to 2%) as well as a diversity of formulations. A key aspect of these efforts is the attempt to identify a therapeutic “sweet spot” that can suppress inflammation and reduce symptomatic recurrences. When used at relatively high (1% to 2%) concentrations, the drug can effectively ameliorate signs and symptoms of severe VKC; at 2%, cyclosporine was shown to be as effective as the corticosteroid dexamethasone (0.1%).11,12 However, both of these studies showed that such brief, high-dose therapy had no significant effect on recurrence following cessation of therapy. Higher concentrations of cyclosporine are thought to increase the probability of epithelial cell damage, so without long-term benefit the risk of using such high concentrations of the drug may not be warranted.
Several other studies employed lower dose regimens of cyclosporine. In one such trial, follow-up at two years showed a reduction in seasonal recurrences of VKC with 0.05% cyclosporine.13 In the same study, however, a higher dose, 0.1%, wasn’t as effective as dexamethasone at treating acute signs and symptoms. However, another study employing similar concentrations of the drug (0.05% and 0.1%) in a different formulation showed that both concentrations provided statistically significant improvement in both corneal staining and subjective symptoms for acute VKC following one month of treatment. (Amrane M, et al. IOVS 2011;52:ARVO e-abstract 6415)
Collectively, these studies show that given the proper formulation, a low-dose anti-inflammatory such as cyclosporine may provide relief for both acute VKC and the recurrences of the disease that can lead to such devastating long-term consequences for ocular health.14
Is VKC a Model for Allergy?
To paraphrase our earlier assessment: When it comes to ocular allergies, one drug does not treat all. And as we learn more about the defining features of the rogues in our gallery, we discover why some drugs work well for some conditions and not for others. So it is with VKC. While many practitioners in the United States may never see a patient with this disorder, understanding the pathophysiology of VKC has provided us clues to properly diagnose and treat all ocular allergies.
Cromolyn sodium, the archetypical mast cell stabilizer, was introduced first for VKC in the 1970s, and was later used for patients with seasonal allergic conjunctivitis.15 We now know that this drug has a slow prevention-based mechanism of action and so is suited to VKC, but is not an effective therapy for seasonal allergies (particularly when compared with topical antihistamines). Despite this, mast cell stabilizers are still being prescribed for seasonal allergies by some clinicians.
This brings us back to the misconception that all ocular allergies are variations of the same disease. We do know that all ocular allergies—seasonal, perennial, atopic, iatrogenic and vernal—share a mixed immunological and inflammatory etiology. But despite these similarities, it’s the differences between them that provide the most useful clues in the search for improved therapies. So while seasonal allergy and AKC are the two conditions with the most prominent immunological etiology, their treatments are completely different. Season allergies respond well to antihistamines, while AKC requires aggressive corticosteroid therapy. When we fully understand the nuances that make perennial allergy different from AKC or VKC, then we’ll be in a position to deliver the optimal therapy for each disease.
REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School and Senior Clinical Scientist at the Schepens Eye Research Institute. Dr. McLaughlin is a medical writer at Ora Inc., in Andover.
1. Kumar S. Vernal Keratoconjunctivitis: A Major Review. Acta Ophthalmol 2009;87:133-147.
2. Bonini S, Coasssin M, Aronni S, Lambiase A. Vernal Keratoconjunctivitis. Eye 2004;18:345-351.
3. Leonardi A, Fregona IA, Plebani M, Secchi AG, Calder VL. Th1 and Th2-type cytokines in chronic ocular allergy. Graefe’s Arch Clin Exp Ophthalmol 2006;244:1240-1245.
4. Bremond-Gignac D, Donadieu J, Leonardi A, et al. Prevalence of vernal keratoconjunctivitis: A rare disease? Br J Ophthalmol 2008;92:1097-102.
5. Abelson MB, Leonardi AA, Smith LM, et al. Histaminase activity in patients with vernal keratoconjunctivitis. Ophthalmology 1995;102:12: 1958-63.
6. Sacchetti M, Lambiase A, Mantelli F, et al. Tailored approach to the treatment of vernal keratoconjunctivitis. Ophthalmology 2010;117:7:1294-9.
7. Abelson MB, ed. Allergic diseases of the Eye. Philadelphia: WB Sauders, 2000:179-196.
8. Abelson MB, Butrus SI, Weston JH. Aspirin therapy in vernal conjunctivitis. Am J Ophthalmol.1983;95:4:502-5.
9. Kumar S, Gupta N, Vivian AJ. Modern approach to managing vernal keratoconjunctivitis. Curr Allergy Asthma Rep 2010;
10:3:155-62.
10. Ohashi Y, Ebihara N, Fujishima H,et al. A randomized placebo controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J. Ocul. Pharm. Ther 2010;
26:165-173.
11. De Smedt S, Nkurikiye J, Fonteyne Y, et al. Topical ciclosporin in the treatment of vernal keratoconjunctivitis in Rwanda, Central Africa: A prospective, randomised, double-masked, controlled clinical trial. Br J Ophthalmol 2011 Oct 14. [Epub ahead of print]
12. Tesse R, Spadavecchia L, Fanelli P, et al. Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol 2010;21(2 Pt 1):330-5.
13. Lambiase A, Leonardi A, Sacchetti M, et al. Topical cyclosporine prevents seasonal recurrences of vernal keratoconjunctivitis in a randomized, double-masked, controlled 2-year study. J Allergy Clin Immunol 2011;128:4:896-897.
14. Restasis Package insert, revised 02/2010. Allergan, Inc. Irvine, Calif.
15. Easty D, Rice NS, Jones BR. Disodium cromoglycate (Intal) in the treatment of vernal kerato-conjunctivitis. Trans Ophthalmol Soc U K. 1971;91:491-9.