Multifocal choroiditis was first described in 1984 in a group of 28 patients with uveitis and lesions at the level of the RPE and choriocapillaris.1 Later, another 28 patients with chorioretinal lesions and vitreous cells were described with what was termed "pseudo-POHS" because the chorioretinal lesions resembled those observed in patients with presumed ocular histoplasmosis.2 In contrast to patients with POHS, however, the patients in these two groups had associated vitreous inflammation. Punctate inner choroidopathy was initially described in 1984 as being characterized by yellowish spots located at the level of the retinal pigment epithelium and inner choroid, most often located in the posterior pole.3
 |
 |
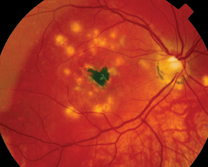
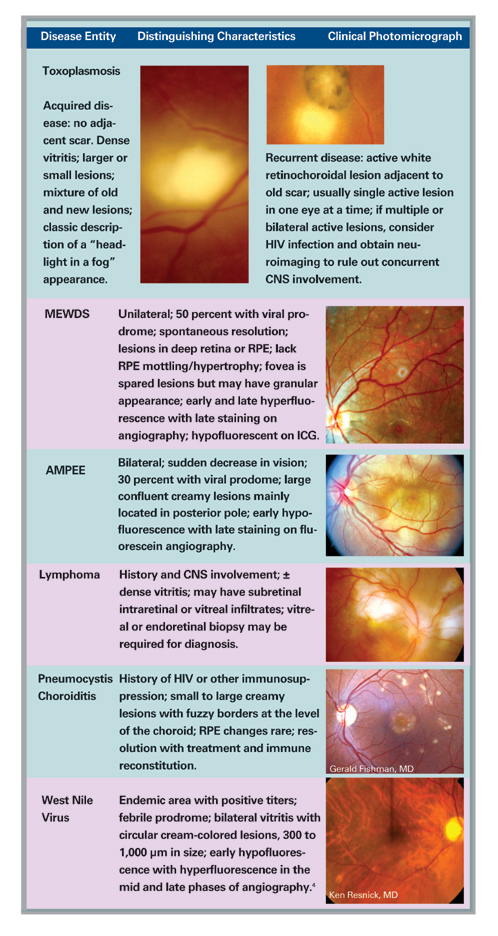
| Figure 1: Fundus photograph demonstrating lesions characteristic of multifocal choroiditis. Note the "pseudo POHS" appearance of the lesions with peripapillary atrophy and RPE changes. Also note the subfoveal fibrosis secondary to choroidal neovascularization. | |
These two distinctly different entities share many similar characteristics and clinical signs, and some authorities consider punctate inner choroidopathy a variant of multifocal choroiditis. It is our goal to demonstrate the similarities and differences between these two entities; and give the reader guidelines that may help to distinguish these two disease processes.
Epidemiology and Clinical Signs
Multifocal choroiditis (MFC) presents most frequently in female patients with an age range of 6 to 69 years, with the average age of presentation in the fourth decade of life. Most patients are moderately myopic, and the majority of patients are affected bilaterally. Punctate inner choroidopathy (PIC) also has a predilection for myopic female patients, but it tends to affect somewhat younger patients, with a mean age of onset of 26 years (range 16-40 years).
MFC patients may present with symptoms of posterior uveitis including decreased visual acuity, floaters, photopsias, as well as with anterior segment symptoms such as photophobia. Almost all patients will present with vitreous cell and approximately 50 percent of patients will have anterior chamber cell.
Funduscopic findings consist of yellow to gray lesions at the level of the RPE and choriocapillaris. The lesions range in size from 50 to 1,000 µm and have a distribution in the peripapillary region, within the arcades, and in the mid-periphery. The lesions may be easily confused with those of POHS. Active lesions may be associated with subretinal fluid and fluffy borders. Active disease may be also associated with optic nerve head hyperemia and edema, cystoid macular edema and, in 25-39 percent of patients, macular and peripapillary choroidal neovascularization. Older inactive lesions are generally punched-out scars with variable pigmentation.
 |
Patients with PIC will present with blurred vision, central or paracentral scotomas, and photopsias. Floaters are not a prominent symptom. The anterior chamber and vitreous tend to be quiet. Funduscopic findings consist of yellow lesions with indistinct borders that measure 100 to 300 µm in size and are located in the posterior pole or mid-periphery. Active lesions may be associated with small amounts of subretinal fluid or serous retinal detachment. Occasionally the optic nerve is hyperemic, but CME does not tend to occur. Old lesions develop a punched-out appearance. Subretinal neovascularization occurs in 25 percent of eyes with PIC.
MFC tends to be characterized by a chronic course or multiple exacerbations and remissions. PIC, in contrast, tends not to recur.
Active lesions in MFC generally demonstrate early hypofluorescence with late staining and leakage on fluorescein angiography. Old scars demonstrate window defects with early hyperfluorescence, well defined borders and late fading. Active PIC lesions, in contrast, demonstrate early mild hyperfluorescence with leakage in the late frames.
Indocyanine green angiography may provide more useful information. Fifty percent of affected eyes with MFC demonstrate hypofluorescent round spots, 200-500 µm in size in the posterior pole. These areas of hypofluorescence do not typically correlate to the lesions that are seen clinically. There is less data available to clearly define ICG findings in PIC, but there appear to be hypofluorescent lesions that correspond to those seen clinically. Patients with MFC may have normal, moderately reduced or severely reduced ERGs.
Differential Diagnosis and Workup
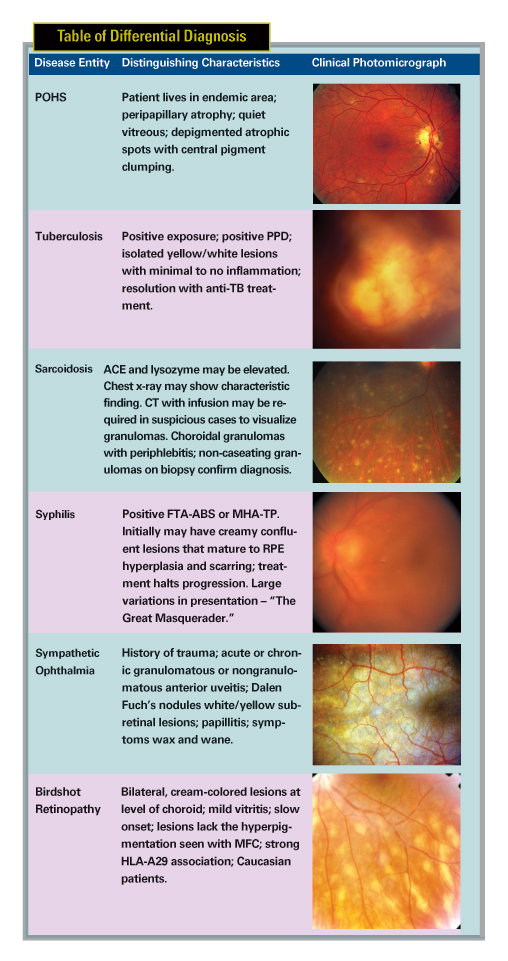
The differential diagnosis for MFC and PIC can be extensive (See Table of Differential Diagnosis). One must have an open mind to both infectious and inflammatory conditions that may present with similar clinical presentations.
Astute observations and a careful history will help guide the workup to avoid a "shotgun" approach to laboratory and diagnostic testing. One must be vigilant to exclude infectious causes of disease prior to pursuing aggressive immunosuppressive therapy. Workup may include FTA-ABS or MHA-TP to rule out syphilis, PPD to exclude tuberculosis, and ACE and lysozyme along with chest x-ray or spiral chest CT to rule out sarcoidosis. HLA-A29 testing may be indicated if there is concern of birdshot chorioretinopathy. Lyme and West Nile Virus titers may be prudent in endemic areas where there is a suggestive history, or supportive symptoms or signs of past infection.
Treatment
Once the clinical diagnosis is established and infectious causes are clearly ruled out, treatment is predicated upon visual acuity and level of ocular inflammation. Treatment for MFC is usually indicated by the presence of CME, dense vitritis, or choroidal neovascular membrane (CNVM) formation. Since there tends not to be inflammatory cells or CME present, PIC is usually not treated except for CNVM.
Treatment may be initiated with topical Pred Forte drops and oral prednisone. In our experience non-generic Pred Forte is more efficacious than the generic formulation. Oral corticosteroids are not without significant side effects, including risk of systemic hypertension, diabetes mellitus, hypercholersterolemia, weight gain/ obesity, insomnia, anxiety, and osteopenia.
Oral prednisone may be started at a moderately high dose (1 mg/kg) and tapered with clinical reduction in inflammation. We try to taper oral corticosteroids quickly and we use more aggressive local therapy including posterior subtenon corticosteroid injection. By using topical corticosteroids for at least three to six weeks prior to subtenon's corticosteroid injection, one may gauge the patient's risk of developing corticosteroid-induced ocular hypertension. Elevated intraocular pressure with topical exposure to corticosteroid drops is a relative contraindication to subtenon's corticosteroid injection. Kenalog injections, 0.5 cc to 1.0 cc (20-40 mg/cc) may be given every one to three months dependent upon the clinical response.
In cases that are refractory to corticosteroids, or in whom corticosteroids cannot be tapered to acceptable levels (5-10 mg p.o. daily by six months) other immunomodulatory agents may be used, including methotrexate, azathioprine, cyclosporine, anti-TNF agents and chlorambucil.5 These agents should be used with great caution, however, as they may have serious and life threatening side-effects. Methotrexate may cause leukopenia and liver damage; cyclosporine use may cause kidney failure; and chlorambucil may lead to significant thrombocytopenia and complete bone marrow failure.
 |
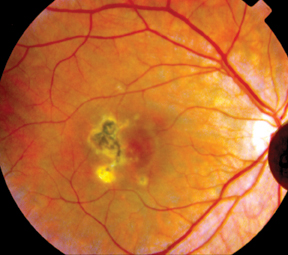 |
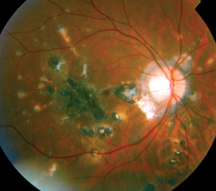
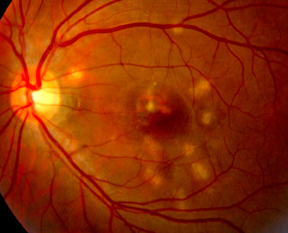
| Figure 2: Fundus photographs demonstrating choroidal lesions associated with punctate inner choroidopathy. A. Active lesions of PIC in the posterior pole of a 29-year-old Caucasian woman. B. Inactive lesions in the right eye of a 28-year-old woman with PIC. The lesions did not demonstrate any changes over a seven-year period. |
MFC and PIC are also associated with choroidal neovascularization. These lesions have been successfully treated with traditional thermal laser and with photodynamic therapy8,9 often together with corticosteroids and non-corticosteroid immunosuppressive agents. Of course, the nature and location of these lesions have a strong impact upon visual outcome.
Prognosis and Outcomes
The long-term visual prognosis of both MFC and PIC is variable and is largely dependent upon the development of refractory CME and CNVM. Visual acuity of 20/40 or better was achieved in 66 percent of eyes with MFC in one series with 95 percent of patients maintaining 20/40 or better vision in at least one eye.10 Twenty-one percent of eyes had worse than 20/200 vision secondary to subfoveal CNV, CME or foveal RPE atrophy. The visual prognosis of eyes with PIC is better, with 75 percent of eyes retaining 20/25 or better vision. The main cause for loss of vision in eyes with PIC is subfoveal RPE atrophy and subfoveal CNVM.
MFC and PIC share many clinical characteristics and signs. However, MFC is a chronic disease that typically demonstrates recurrences and may require long-term immunotherapy. PIC typically does not recur, has a better prognosis and typically does not require treatment except for CNVM. Distinguishing between these two entities is important, in order to counsel patients and develop treatment plans. We also stress the importance of long-term follow-up and compliance, especially when steroids and immunosuppressive agents are utilized. With these precepts set forth, we find treating these patients can be a very rewarding experience.
Dr. Goldstein is an associate professor of ophthalmology and co-director of the Uveitis Service at the University of Illinois Eye and Ear Infirmary. Contact her at debrgold@uic.edu. Dr. Ulanski is a clinical fellow in uveitis at the University of Illinois Eye and Ear Infirmary, and in private practice with Vitreo-Retinal Consultants, Detroit, MI. Contact him at LJUII@cs.com.
1. Dreyer RF and JDM Gass. Multifocal choroiditits and panuveitis: a syndrome that mimics ocular histoplasmosis. Archives of Ophthalmology 1984;102:1776-1784.
2. Deutch TA and HH Tessler. Inflammatory pseudohistoplasmosis. Annals of Ophthalmology 1985;17:461-465.
3. Watzke RC, Packer AJ, Folk JC, Benson WE, Burgess D, and RR Ober. Punctate inner choroidopathy. Am J Ophthalmology 1984;98:572-584.
4. Vandenbelt, S, Shaikh, S, Capone A, and G Williams. Multifocal choroiditis associated with west nile virus encephalitis. Retina 2003;23:97-99.
5. Goldstein DA, Fontanilla FA, Kaul S, Sahin O, and HH Tessler. Long-term follow-up of patients treated with short-term high-dose chlorambucil for sight-threatening ocular inflammation. Ophthalmology 2003;109(9):370-377.
6. Samson CM, Waheed N, Baltatzis S, and CS Foster. Methotrexate therapy for chronic non-infectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001; 108(6): 1134-1139.
7. Kaplan-Messas A, Barkana Y, Avni I, and R. Neumann. Methotrexate as a first-line corticosteroids-sparing therapy in a cohort of uveitis and scleritis. Ocular Immunology and Inflammation 2003; 11(2): 131-139.
8. Spaide RF, Freund KB, Slakter J, Sorenson J, Yannuzzi LA and Y Fisher. Treatment of subfoveal choroidal neovascularization associated with multifocal choroiditis and panuveitis with photodynamic therapy. Retina 2003;22(5):545-549.
9. Wachtlin J, Heimann H, Behme T, and MH Forester. Long Term results after photodynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefe's Arch Clin Exp Ophthalmol 2003;241(11):899-906.
10. Brown, J., Folk, JC, Reddy, CV, and AE Kimura. Visual prognosis of multifocal choroiditis, punctate inner choriodopathy, and diffuse subretinal fibrosis syndrome. Ophthalmology 1996;103: 1100-1105.