Optical coherence tomography angiography is a convenient and noninvasive way to identify and follow retinal and choroidal vascular pathologies by providing cross-sectional and three-dimensional images. However, despite these benefits, more work needs to be done before OCTA can be used in all patients, according to experts.
“OCTA has been around for several years,” says David Boyer, MD, who is in practice in Los Angeles. “Its acceptance in the community is growing, partly because earlier versions of OCTA had many artifacts that made it difficult for the average ophthalmologist to interpret. Manufacturers have worked at reducing the artifacts, and I view the OCTA as a valuable asset in our armamentarium for examining patients. However, its place is still being worked out, regarding which patients should have it and when.”
Here, physicians experienced in using OCTA discuss its strengths and limitations.
Understanding the Options
For decades, dye-based angiography has been the standard clinical imaging modality for evaluating retinal and choroidal vascular pathologies. Unfortunately, fluorescein and indocyanine green angiography are invasive and time-consuming. Additionally, fluorescein angiography is only two-dimensional and is unable to visualize the deeper capillary structures. Indocyanine green angiography is used mostly to image the choroid.1,2 Because of these limitations, researchers have been studying faster, safer imaging tools that are capable of effectively imaging both the retinal and choroidal circulations.
OCTA makes it possible to study the hemodynamics of individual retinal and choriocapillaris vascular layers noninvasively. A recent review found that OCTA is a useful modality for evaluating retinal and choroidal blood flow in patients with inherited retinal diseases, including retinitis pigmentosa, Stargardt’s disease, Best vitelliform macular dystrophy and choroidemia.3 OCTA imaging has yielded new insights into the occurrence of vascular insufficiency in these conditions. Using OCTA to study retinal and choroidal blood flow in patients with inherited retinal diseases and dry macular degeneration may reveal further insights into the pathogenesis and natural history of disease in these conditions. The currently available OCTA units in the United States are the Spectralis OCTA from Heidelberg, Optovue’s Angiovue and Zeiss’ AngioPlex.
According to Dr. Boyer, the main benefit of OCTA is that it can be applied earlier and in a noninvasive way to monitor disease progression. “Much of this information can be obtained from the fluorescein angiogram, but that’s an invasive test that would need to be repeated,” he says. “OCTA has no radiation. It helps correlate the vascular changes that are occurring before we’re able to visualize them. So, we will have more information on the disease process, basically.”
He adds that the use of OCTA has become more commonplace, and it is used for a variety of conditions. “It’s used for macular degeneration,” he says. “Using OCTA, we can identify drusen that have become vascularized, which we could not do before. We can also see nonexudative choroidal neovascularization. Fluorescein angiography doesn’t allow us to see those things.”
Jennifer I. Lim, MD, director of the Retina Service at University of Illinois Health, adds that speed is a benefit of OCTA over fluorescein angiography. “Additionally,” she says, “it provides 3D spatial localization, so you can see the different layers of the blood vessels, including the choroid, with the available segmentation layer analyses, without overlying vessels, unlike with fluorescein angiography. On fluorescein angiography, the vascular layers are matted on top of each other, so there are overlays. And, because OCTA is noninvasive, it’s safer; you don’t have to worry about allergic reactions to fluorescein dye. It is also helpful for imaging children and people with poor venous access.”
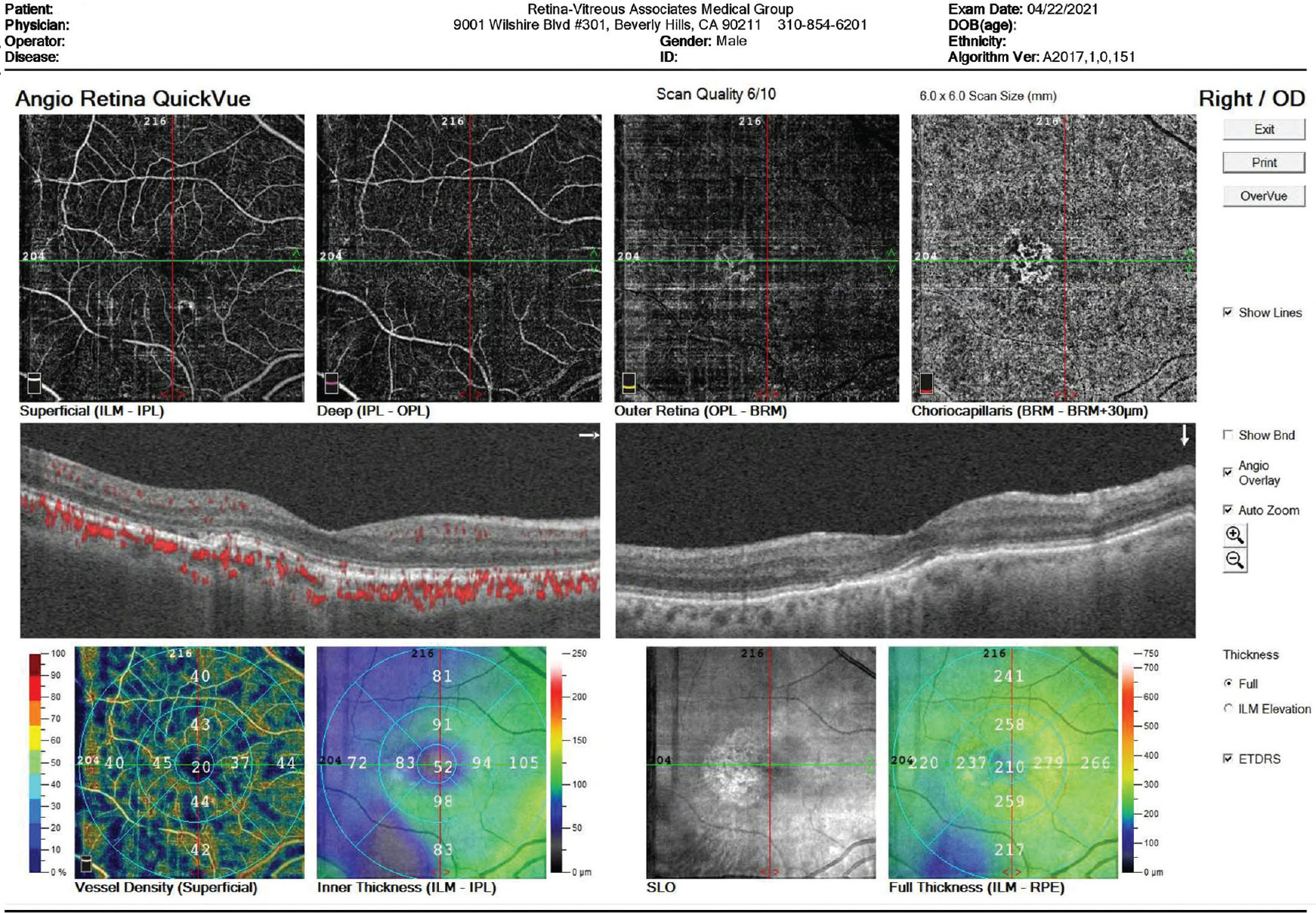 |
| A choroidal neovascular lesion. Physicians say that optical coherence tomography angiography can be useful in such cases because they’re able to see the choroidal neovascular membrane despite the presence of overlying retinal leakage that would obscure its presence on a fluorescein angiogram. (Image courtesy of David Boyer, MD) |
Dr. Lim says OCTA is helpful in particular conditions. “Today, OCTA is useful for identifying choroidal neovascularization,” she says. “If you see the CNV vessels, you know it’s there, and you don’t have to do fluorescein angiography. It’s also nice for follow-up on these patients to see the size of the choroidal neovascular membrane. A second useful indication is to clearly see the abnormal neovascularization of the retina in diabetes.
“It’s also really useful when there is a disease that causes a lot of retinal vascular leakage and you’re trying to determine whether there is an underlying choroidal neovascular membrane,” she continues. “These situations occur when there is central serous chorioretinopathy or uveitis. The OCTA is useful because there’s no leakage seen from the retinal vasculature, so you can visualize the underlying vessels in the OCTA image. You can see the choroidal neovascular membrane despite the presence of overlying retinal leakage that would obscure its presence on a fluorescein angiogram.”
However, OCTA is not without its drawbacks. The images obtained are sometimes blurred and difficult to interpret. “For some images, it may be difficult to know what’s noise or artifact versus what’s real pathology,” Dr. Lim says.
According to Thomas Stone, MD, in practice in Louisville, Ky., most OCTA units don’t provide the same amount of information as dye-based angiograms. “In other words, you receive information about a smaller area, and it doesn’t show leakage,” he says. “It just shows where vessels are open and if there are new vessels, but it doesn’t tell you if the vessels are leaking. It’s not a dynamic view like fluorescein angiography.”
Additionally, it hasn’t yet been determined when OCTA should be used and in which patients. Dr. Stone uses it for his patients with retinal vascular disease and in diabetic patients if they have edema or if their vision has decreased. “It gives me an idea of what their visual potential will be if I give them injections,” he says. “Additionally, for patients with retinal vein occlusions, I can gauge the amount of ischemia, and that gives me a sense of how much treatment the patient will require. I also use it in choroidal neovascular disease.”
Swept-source OCTA offers a wider field of view and can be a valuable tool. “However, this is a specialty item that many people don’t want to invest time and money in,” notes Dr. Stone. “I don’t think OCTA will ever fully replace traditional angiography, because I think there’s still a role for evaluating the leakage.”
He says that, while the use of OCTA is currently not critical, it is helpful. “If I end up in a clinic without it, it’s not like I can’t take care of patients; however, there’s definitely a subset of patients in whom it can help you make more informed decisions about whether to treat them and how to treat them,” he says. “It’s not currently standard of care, but it certainly makes your care better and more thorough in the subset of patients who need it.”
Research and Artificial Intelligence Applications
In addition to clinical applications, OCTA has research and artificial intelligence applications, says Dr. Lim. “Our group is working a lot on artificial intelligence applications to use OCTA for diagnosis of retinal conditions such as sickle cell diabetic retinopathy,” she says. “We’ve shown that you can actually use OCTA, and that the sensitivity and specificity are pretty high based on quantitative parameters.”
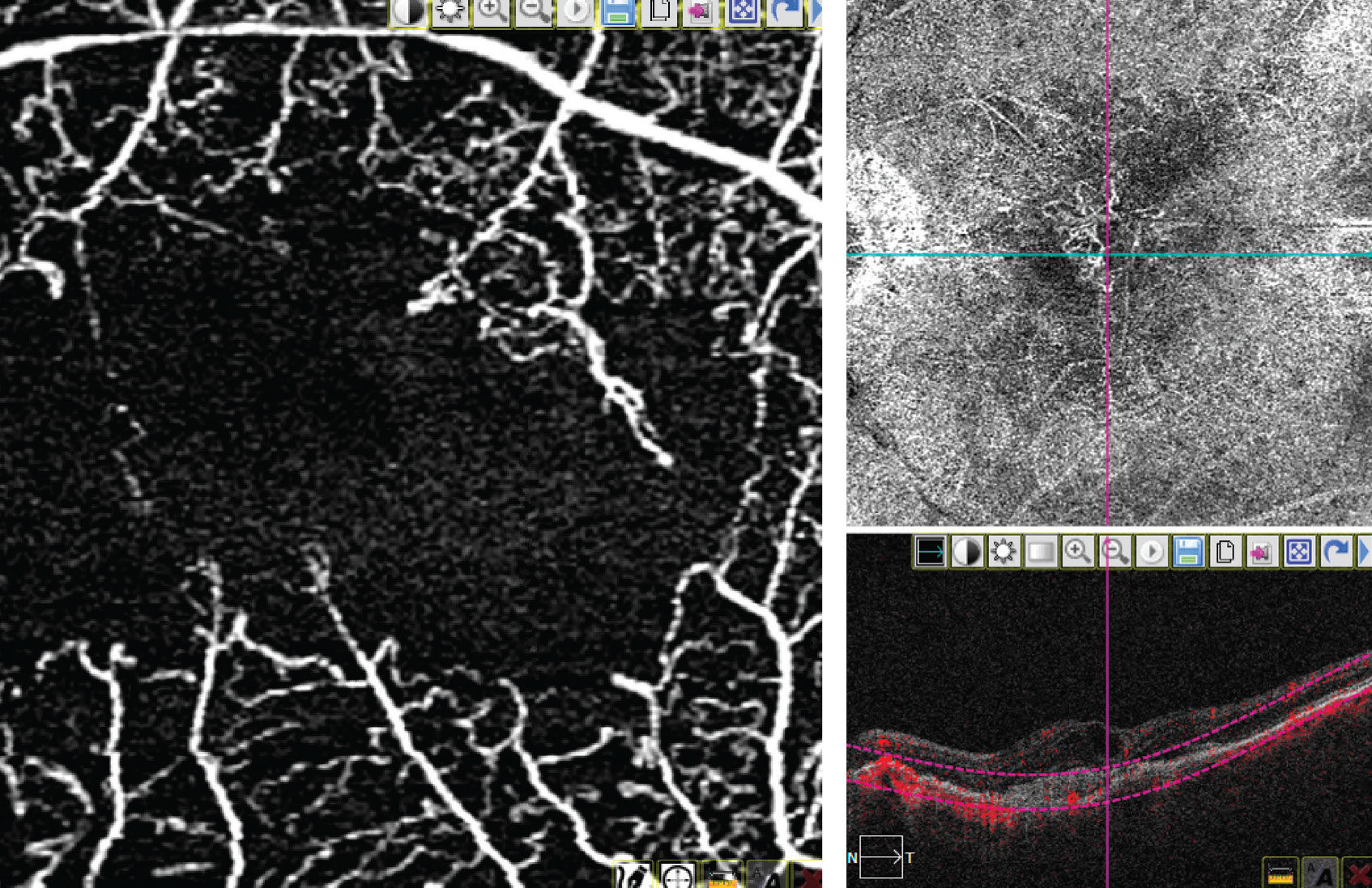 |
| A 3x3 macular image on OCTA (left) and an 8x8 image of the macula on OCTA (right). (Image courtesy of Thomas Stone, MD) |
A recent study conducted by Dr. Lim and her colleagues found that artificial intelligence classification is a promising novel and affordable screening tool for clinical management of ocular diseases.4 The study included an OCTA image database of 115 images from 60 diabetic retinopathy patients (20 mild, 20 medium, and 20 severe cases of nonproliferative diabetic retinopathy), 90 images from 48 sickle cell retinopathy patients (30 patients had stage II mild and 18 patients had stage III severe sickle cell retinopathy) and 40 images from 20 control patients. There were no statistically significant differences in age and gender distribution between the three groups. In patients with diabetic retinopathy, no significant difference in hypertension or insulin dependency between stages of disease groups was observed.
They used a logistic regression-based model with backward elimination to select the optimal combination of features for the multi-task classification. Blood vessel tortuosity, blood vascular caliber and foveal avascular zone parameters increased with disease onset and progression, while blood vessel density and vessel perimeter index decreased. The backward elimination initially started with all OCTA features and eliminated features one by one based on the prediction accuracy of the fitted regression model. The feature selection method identified an optimal feature combination for each classification task.
The support vector machine classifier performed the classification tasks in a hierarchical manner. Then, the investigators measured the sensitivity and specificity task to evaluate the diagnostic performance in each task. The receiver operation characteristics curves were drawn, and the area under the receiver operation characteristics curves were calculated for each task. At the first step, the support vector machine distinguished diseased patients from control subjects with 97.84-percent sensitivity and 96.88-percent specificity. After identifying the patients with disease, the classifier sorted them into two groups: diabetic retinopathy and sickle cell retinopathy, with 95.01 percent sensitivity and 92.25 percent specificity.
After sorting into corresponding retinopathies, the support vector machine conducted the condition staging classification: It had 92.18 percent sensitivity and 86.43 percent specificity for nonproliferative diabetic retinopathy staging (mild vs. moderate vs. severe), and 93.19 percent sensitivity and 91.60 percent specificity for sickle cell retinopathy staging (mild vs. severe).
The Future
Dr. Boyer believes that the use of OCTA will continue to increase. “OCTA allows us to see layers of the retina that we couldn’t see,” he says. “In other words, there are evidently three capillary plexuses, and we can only see one on fluorescein angiography. Now, we’re seeing two. We can see a superficial and a deeper area of capillary circulation and that’s giving us a better idea of where ischemia is occurring. So, I think OCTA is adding to our knowledge of different disease states because we’re now able to visualize areas we couldn’t visualize before.”
Dr. Lim agrees. “In the future, I’d like to think that every retinal specialist would have one in his or her office, if the cost came down enough so that it was affordable. Second, if the noise-to-signal ratio can be improved, I think it probably will become more mainstream,” she says.
She explains that manufacturers are currently working to improve the software. “If you remember, when first-generation optical coherence tomography first came out, people said they would never use it,” she says. “Some pretty famous retina specialists said OCT would never catch on. They said they would never use it because the images were too fuzzy, so you couldn’t tell what’s real and what wasn’t. Then, with the later-generation OCT units, with improved image quality, OCT caught on like wildfire. Now, essentially every retinal specialist has an OCT. I think the same thing will happen with OCTA.”
Drs. Boyer, Lim, and Stone have no relevant financial interests to disclose.
1. DeCarlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). International Journal of Retina and Vitreous 2015;1:5.
2. Weinhaus RS, Burke JM, Delori FC, Snodderly DM. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Experimental Research 1995;61:1-16.
3. Ong SS, Patel TP, Singh MS. Optical coherence tomography angiography imaging in inherited retinal diseases. Journal of Clinical Medicine 2019;8:12:2078.
4. Alam M, Le D, Lim JI, Chan RVP, Yao X. Supervised machine learning based multi-task artificial intelligence classification of retinopathies. Journal of Clinical Medicine 2019;8:6:872.



