For many ophthalmologists, optics is a subject that’s often neglected. I’ve taught review courses in optics for many years, and I’ve become accustomed to having practicing ophthalmologists and residents-in-training from every field of ophthalmology (especially retina, glaucoma and oculoplastics) tell me that they only learned about optics in order to pass their board or recertification exams. “I don’t really need to know optics for everyday use in my field, except on multiple choice tests,” they say. I reply that they’re misinformed. When making important medical decisions, clinical optics may be the key consideration—every bit as crucial as their medical concerns for the patient.
Here, I’d like to provide a few examples of clinical situations all of us encounter from time to time, situations in which optics plays an important role in making the best decision about how to proceed. Hopefully, these examples will serve as a reminder of just how much optical considerations impact our daily decisions as ophthalmologists.
Presbyopic Unilateral Cataract
 |
 |
| This patient has a unilateral cataract OD (acuity 20/70) with +7 D of hyperopia in both eyes. To avoid anisometropia, many surgeons would aim to leave the right eye with +4 D of hyperopia postop; but if the other eye then develops a cataract, the individual will be left hyperopic, unnecessarily. A better approach is to make the right eye plano and have the individual wear a contact lens in the left eye until the left eye needs surgery. The long-term result will then be 20/20 vision in both eyes, with spectacles only needed for near vision. | |
Over the years I’ve heard some surgeons suggest choosing an intraocular lens that leaves the operative eye with +4 D of hyperopia, while the unoperated eye remains at +7 D. What the surgeon is trying to do, of course, is prevent anisometropia—eye strain that can result when there’s a marked difference (usually >3 D) in refractive error between the two eyes. This is generally not an issue when the patient is wearing single-vision spectacles; it’s also not an issue if the patient can accommodate. But if the patient can no longer accommodate (either because of presbyopia or pseudophakia), and needs a bifocal or trifocal lens, anisometropia becomes a potential problem. Because of most people’s inherently small vertical fusional capabilities, the difference between the two prescriptions and the prismatic effect of the reading add lenses can end up inducing vertical double vision when the person engages in near-related tasks. In addition, the patient may experience eye strain as a result of the difference in prescriptions with distance-related tasks. To avoid that problem, most ophthalmologists try not to leave more than 3 D of refractive difference between the eyes. However, this can backfire.
Suppose you decide to use this strategy, avoiding anisometropia by making the patient +4 D in the operated eye. A year or so later, the patient could get an unexpected cataract in the second eye. Now the patient is +7 in one eye and +4 in the eye you’ve already operated on. What do you do to the second eye? Make it +4 D, +3 D, or +2 D to reduce or balance the anisometropia? If so, you’ve committed an optical crime, because when you intentionally leave someone hyperopic, the patient has no clear image at any distance without correction.
A better approach is to make the first eye plano postop; you can prevent anisometropia by having the patient wear a contact lens on the fellow eye that hasn’t yet developed a cataract. (To make sure the patient will tolerate wearing a contact lens, you can have him practice wearing one on the non-operative eye well in advance of the surgery.) Because the vertex distance between the crystalline lens and the corrective lens in the eye wearing the contact lens is now insignificant, anisometropia is no longer an issue. And as a result of following this strategy, if the patient eventually develops a cataract in the second eye, you can make that eye plano as well. Both eyes end up with superb uncorrected distance vision, and all the patient will have to do is wear reading glasses for close work. This is a long-term win-win scenario for the patient.
This optical concept can also be applied if the patient in this hypothetical case scenario were highly myopic in both eyes at the outset. It’s true that ending up with both eyes a little myopic is less problematic than leaving a patient with both eyes hyperopic; the myopic patient will still have some usable near vision without spectacles. But making the first eye plano and placing a contact lens on the fellow eye until it needs cataract surgery has the long-term benefit of leaving the patient with superb uncorrected distance in both eyes after the second surgery. I would add that having a patient learn to use a contact lens in one eye can open him up to the possibility of future monovision, via a monofocal IOL, when the second eye requires cataract surgery.
Note: I would advise against performing LASIK to correct the fellow (clear) eye in either the hyperopic or myopic scenario. As we all know, a cataract—such as a posterior subcapsular cataract in a younger patient or diabetic—can sometimes rapidly occur in the second eye.
Vitrectomy vs. Scleral Buckle
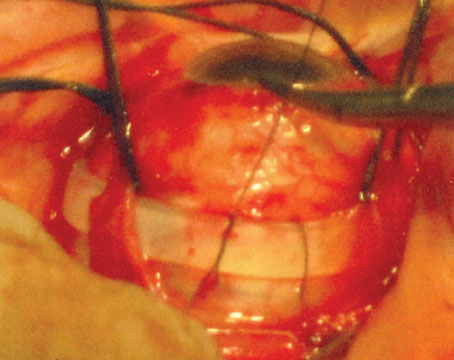
Suppose you’re a retina specialist and a patient comes into your office with a retinal detachment in an eye that contains an IOL. The eye needs surgical repair. The question is, should you perform a vitrectomy or use a scleral buckle?
 |
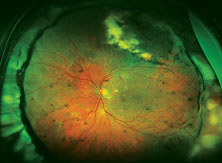 |
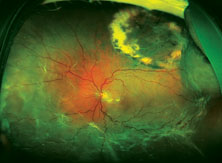
| Top: Panoramic view of the retina in an eye with an inferior retinal detachment extending from the 3 o’clock to 9 o’clock periphery. Bottom: The reattached retina with a scleral buckle encircling band. |
The reason optics is so important in this situation is that using a scleral buckle enlarges the eyeball and increases the axial length, inducing anywhere from 1 to 4 D of refractive myopia (See photos, p. 68). In fact, you’ll create anisometropia if you choose a scleral buckle. So, if you choose this option, the patient needs to be warned that his prescription will change. He may have double vision when seeing up close if he uses presbyopic corrective lenses; he may need to wear a contact lens on this same eye to reduce anisometropia; and he may have to get a special reading lens, such as a slab off (or reverse slab). Furthermore, the situation is complicated if the IOL in the eye being repaired for a retinal detachment is multifocal. By making the patient more myopic you’ll eliminate the multifocal IOL’s ability to correct at all ranges.
For all of these reasons, if you have a choice between vitrectomy and scleral buckle, it’s important to consider the optical consequences of choosing the buckle. If those optical consequences will be severe—as they would in this case—I’d opt for vitrectomy to avoid inducing myopia. Obviously, if the patient has multiple detachments, or the situation requires a buckle, then retinal issues take priority over the optical concerns. If that’s the case, the patient needs to be informed of the anticipated anisometropia that will be caused by the scleral buckle, and any loss of optical benefits from the IOL.
If the patient has a multifocal lens in the operative eye, you could, in theory, exchange it for a monofocal lens in the future if there are any unwanted optical side effects. Unfortunately, doing an elective procedure to exchange an IOL in an eye that’s had a prior retinal detachment is really not a good idea; any additional procedures in that eye could trigger additional retinal pathology and complicate the patient’s eyesight.
The bottom line is that if you find yourself in this situation, make sure you take into account the optical consequences of your choice. It’s worth discussing these considerations with the cataract surgeon as well. Many retinal surgeons are accustomed to going it alone, but in a situation like this one you’re most likely to end up with a happy patient if you involve the cataract surgeon (who will also probably refer more patients to you in the future). In addition, as the retina specialist, you can take this opportunity to discuss with the cataract surgeon your everyday frustrations relating to repairing retinal detachments in eyes with silicone IOLs.
Mixing IOL Materials
Suppose a patient with a monofocal acrylic IOL requests a specific pseudo-accommodating IOL in the second eye—one that’s composed of silicone. Given the different materials, should you implant this IOL in the fellow eye? Proceed with caution.
 |
 |
| Views of a painting seen through the right and left eyes of the same patient with a different IOL material in each eye. The difference is the result of chromatic aberration caused by the different lens materials. | |
At first we were stumped. Both lens implants looked pristine and both eyes were completely healthy. But then it dawned upon us to look at the IOL ID cards in her purse that she received with each surgery. It turned out that one eye now had an acrylic lens while the other eye had a silicone lens.
The problem that arises in this situation is chromatic aberration. Acrylic has a higher index of refraction than silicone, so the light travels more slowly and is refracted differently when it passes through silicone than when it passes through acrylic. That actually gives acrylic lenses an advantage, because they have a higher index of refraction and have the ability to “bend” light more. This means that a lens made of acrylic will produce a higher diopter power than a silicone lens of the same thickness. That’s why you’ll see IOL powers up to 40 D in acrylic lenses, while a silicone lens may only be available up to 30 D.
The result of having a different index of refraction in each eye is that colors look different in each eye. Of course, many patients will tolerate this difference; some might not even notice it. But certain patients, such as artists, photographers or pilots who depend on color vision for their livelihood, will notice it right away.
Every photographer is familiar with chromatic aberration. It’s a problem when you’re taking an outdoor photo with numerous colors in the picture, such as a cluster of different-colored balloons. Even if all the balloons are theoretically in focus, some will appear out of focus relative to the others because of chromatic aberration. Light travels faster at certain color wavelengths, as well as when traveling through different materials. That’s why professional sports photographers use high-end camera lenses that cost thousands of dollars; the lenses minimize chromatic aberration.
The bottom line is that you should avoid mixing and matching different materials in one patient. If a patient came into our office requesting a lens made of a different material from the lens in his fellow eye, I’d dissuade him from getting the lens and explain the issues surrounding chromatic aberration, to make sure he could make an informed decision.
This is one reason patients are given an implant card at the time of cataract surgery. In the event the patient has a second cataract surgery done by a different surgeon (if the patient moves to a different city, for example), the second doctor will know the identity of the first implanted lens and have the opportunity to match the material and model to achieve optical and chromatic balance.
End-stage AMD & Cataracts
Suppose a retired librarian comes in with end-stage macular degeneration, bilateral macular scars and bilateral cataracts, with a BCVA of 20/100. Should you aim to leave the patient plano, myopic or hyperopic after cataract surgery?
If the librarian does a lot of reading and needs to see things up close, you should aim to leave him a little bit myopic—around -1.5 to -3 D, depending on his preferred reading distance and arm length. Why? Because by leaving the patient with about 2 D of myopia, the patient can now buy an inexpensive over-the-counter +2- to +3-D pair of reading glasses, and the combination will provide the full amount of magnified near vision the patient needs for near work. In essence, you’ve converted an inexpensive pair of over-the-counter reading glasses into a powerful low-vision aid for someone who would potentially be blind without it.
How do you determine where you want the near point of accommodation to be for a given patient? Kestenbaum’s rule (for determining the necessary near add in a patient with compromised acuity) states that the inverse of the patient’s best Snellen visual acuity is the number of diopters of add required. In this case, the inverse of 20/100 is 100/20, or 5 D. The 2 D of myopia you’ve left the patient with, plus 3-D OTC reading glasses, can now provide the patient with the near vision he needs.
Why is this so important? Consider the alternative: If you’d made the patient plano in both eyes, targeting for distance, he’d need +5 D reading glasses. As any optician will tell you, a pair of +5 D reading glasses is expensive to manufacture. You’ll have to put prism in the glasses to bring the images into alignment, because looking through +5 D spectacles without prism will cause diplopia at near—the brain will require excessive fusion to merge the two images. By intentionally making the patient myopic at the time of cataract surgery, you’ve turned an inexpensive device into a powerful low-vision aid, improved the patient’s quality of life and reduced the economic burden for the patient and society—all because you took optics into consideration.
By the way, this concept of providing a near add by making the patient myopic with the monofocal IOL won’t benefit patients who have end-stage glaucoma. In fact, creating a low-vision magnifying system with near-AIM lens implants can do an end-stage glaucoma patient an optical injustice. These individuals have often lost their peripheral vision and only have a small central field of vision. Magnifying letters or images will only enlarge them into the peripheral scotoma and thus offer no benefit.
Returning to our librarian with end-stage macular degeneration, I’ve heard the argument that it would benefit the patient to make the eyes hyperopic with the lens implants. This supposedly creates a telescopic system that allows the patient to see at distance with a corrective lens. As in the first case described earlier, I would argue that it’s an optical crime to intentionally make any patient hyperopic. Doing so causes all images to be focused behind the retina, so the patient has no clear focus regardless of the distance of an object from the eye. (I’ve discussed this with many ophthalmologists, vision scientists and optometrists, and they all agree.)
If you’re still not convinced, you can easily determine whether hyperopia is beneficial by creating hyperopia in your own eyes using contact lenses, when your accommodation has been neutralized pharmacologically or because of your age. You’ll see firsthand how optically miserable hyperopia can be—there’s no clear image in focus at any range, near or far.
No human eye should ever intentionally be made hyperopic.
Irregular Corneas and Toric IOLs
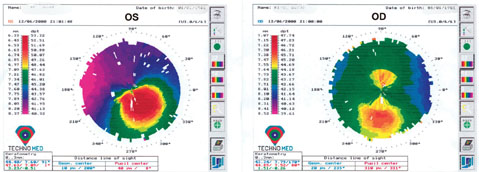 |
| Keratoconus (left image) and forme-fruste keratoconus (right image – asymmetric bowtie) noted on topography. This patient would not be an ideal candidate for a toric lens implant in either eye. Note: It’s important to compare corneal topographies in both eyes as the other eye can offer insight to any underlying pathology—in this case, subclinical keratoconus in the right eye. |
In this situation, you need to consider two key things. First, is the patient fully healed from the corneal transplant? Are there any additional sutures to be removed that might help minimize the astigmatism? Second, if the patient is fully healed and still has persistent astigmatism: Is the astigmatism regular or irregular? If you see a bow tie pattern in any axis on topography, you most likely have regular astigmatism. If you see anything that deviates from the classic bow tie pattern, you most likely have irregular astigmatism.
As you know, a toric lens implant is designed to correct regular astigmatism. I would use caution when deciding to put a toric lens in one of these patients, because if the patient turns out to have a little bit of irregular astigmatism and the axis of the lens rotates unexpectedly or isn’t placed correctly, you’re going to create a complex optical situation with irregular astigmatism at the cornea and lenticular astigmatism at the lens plane. The resulting refractive astigmatism will be impossible to correct using either spectacles or a contact lens—hard or soft.
For that reason, in this situation I’d argue that it’s best to err on the side of being conservative. I’d put in a standard monofocal lens. Then, should the patient have any kind of irregular corneal astigmatism postoperatively, that can be corrected with a rigid contact lens.
The Upside-down Lens Implant
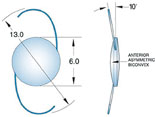 |
 |
|
Above left: A multi-piece IOL properly aligned in an eye during surgery when viewed with the patient supine produces the "Z-shaped configuration" and 10 degrees of angulation of the haptic-optic plane. Above, middle: A single-piece IOL proper aligned in an eye during surgery, viewed with the patient supine. There should be no angulation of the haptic-optic plane. Above right: This multi-piece acrylic lens implant was placed upside down, displaying an "S-shaped configuration." The IOL has prolapsed anteriorly into the sulcus because of the negative angulation of the lens haptic-optic plane. (A superior laser iridotomy was placed to relieve an attack of pupillary-block angle closure glaucoma.)
|
|
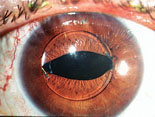
For example, we had a patient many years ago who was referred to us for an attack of angle-closure glaucoma. The attack occurred after a routine dilated eye exam to check his status after cataract surgery. After we reduced the patient’s IOP medically and cleared the corneal edema, we were able to determine that the lens implant had been placed upside down.
Suppose you’re faced with an eye in which an intraocular lens has been placed upside-down. Should you risk damaging the corneal endothelium and/or the capsular bag to correct this?
The answer depends on the type of lens implant that was inserted. For example, is the lens a single-piece implant, or a multi-piece implant such as the three-piece Alcon MA60AC or Tecnis ZA9003? If it’s a multi-piece implant, or any IOL that has separate haptics and optics, the eye could be in trouble. These implants have angulation—the optic-haptic plane is not flat; it’s angled or bent. This is intended to make the implant vault backwards in the direction of the posterior chamber when properly inserted into the capsular bag, for stability. If placed upside-down, the implant can vault forward out of the capsule bag and into the sulcus (See photo, above right).
This can have two serious consequences. First, changing from a posterior vault to an anterior vault will induce unintended myopia. Second, the anterior vault can cause pupillary block angle-closure glaucoma, especially if the pupil is dilated for a routine exam and then constricts once the dilation has worn off (as happened in the patient who was referred to us). For these reasons, multi-piece IOLs cannot be left upside-down; they have to be placed in the correct configuration—the so-called Z-shaped configuration. When the patient is facing you and you look into the eye, you should see the implant and optics arranged in the Z-shaped configuration (See illustration, above left). If you see the “S-shaped configuration,” then the IOL is placed upside down.
If the implant is a single-piece IOL such as the Alcon SN60WF, the Softec HD lens or the Tecnis ZCB00, you won’t have either of these problems because these implants have zero angulation; the lens-haptic plane is flat. Thus, these eyes are not at risk for pupillary-block glaucoma after dilation because the IOL will stay in the capsular bag. However, whether the patient’s intended postoperative refraction will be altered by the upside-down configuration or not depends on the construction of the implant.
If the upside-down implant is an aspheric IOL such as the SN60WF or the Tecnis ZCB00 lenses, you could end up with an optical surprise because the front surface is more aspheric (steeper) than the posterior surface. This could result in a different postoperative refraction from your intended result. You also lose the benefits of the lens asphericity (reduced spherical aberration at night because of large pupil sizes) if the single-piece IOL is placed upside-down. On the other hand, if you have a bi-aspheric lens such as a Softec HD IOL, you can put the lens in upside-down and you won’t have any optical problems because both surfaces are identical.
Of course, the best way to handle this situation is to prevent it in the first place by making sure you’re inserting the lens right-side-up. The take-home point is to completely understand the physical, geometric and optical properties of the lens implants you insert. Take the time to read those mandatory FDA package inserts that come with every lens. That way, if there’s a problem, you’ll know why it happened and what to do to correct it.
Tolerating a Multifocal IOL
| Rigid Contact Lenses: An Ophthalmologist’s Best Friend |
|
Unfortunately, irregular astigmatism is a common problem with penetrating keratoplasty patients. Even with the best femtosecond laser-assisted corneal transplant techniques, it's still not possible to suture a cornea to an eye and end up with completely normal curvature; there will always be some areas that end up uneven, producing irregular astigmatism. That's why a rigid gas permeable contact lens should be every corneal transplant surgeon's best friend. A rigid lens eliminates irregular corneal astigmatism and surface abnormalities to an extend that not even high-definition wavefront excimer lasers can match. That makes it an excellent tool for enhancing a patient's vision after a corneal transplant.
The same principle applies to non-transplant patients who have keratoconus or ectatic corneal conditions such as post-LASIK complications or pellucid marginal degeneration. These patients also have an irregular corneal surface that doesn't produce the bow tie pattern on topography, and these are patients in whom you should not put a toric lens. However, a rigid contact lens will help these patients, as it does PK patients; it smoothes the cornea and balances out tear-film irregularities to create a clear image.
Because a hard lens could turn out to be a powerful aid for a PK patient, I recommend having these patients practice wearing one before you operate. This could save you and the patient some unpleasantness afterwards, should the patient find the lens intolerable. (At the same time, you shouldn't let the patient give up on these lenses too easily; many patients adapt to them over time, despite initial discomfort.) If you find out beforehand that the patient will have trouble tolerating a rigid lens, you can also consider one of the new hybrid contact lenses, which combine a hard center with a soft periphery. These provide the comfort of a soft lens but the optical clarity of a hard contact lens. - Tommy Korn, MD, FACS
|
When it comes to implanting multifocal IOLs, the million-dollar question is: Will the patient be able to tolerate the optical side effects?
Clearly, not everyone does, which isn’t surprising given that the brain is accustomed to receiving a single, clear image. Yes, some patients are thrilled with the advantages of multifocal vision, but there are also numerous patient blogs and websites devoted to complaints about halos, glare, loss of contrast sensitivity and trouble driving at night. To put it simply, some patients who have received multifocal lens implants are miserable. So: How do you determine who will be happy with a multifocal lens? If there were a simple way to figure this out before cataract surgery, a lot of patient unhappiness might be prevented.
It’s worth noting that, generally speaking, a patient who has a dense cataract and who’s had lifelong myopia or hyperopia is more likely to be happy with whatever type of lens you put in. A hyperopic or myopic patient is accustomed to the optical aberrations from thick spectacles, spherical aberration or chromatic aberration from high-index spectacles. These patients have never had a clear image at distance without glasses, so they may be more willing to tolerate any halo, glare or loss of contrast sensitivity that comes with a multifocal IOL, as long as you provide them with a clear image at distance. But you should not interpret enthusiasm from a patient like this to mean that multifocal lenses are the best thing for every cataract patient you see.
In my experience, if you do cataract surgery on a patient with a mild or moderate cataract who’s also been a lifelong emmetrope and never worn glasses except for presbyopia, that patient will most likely be very hard to satisfy with a multifocal lens. A patient like this will be accustomed to having good vision at distance, which will now be compromised somewhat by the multifocality of the new lens implanted in his eye with its potential for halo, glare and loss of contrast sensitivity.
One strategy that may help is to be proactive when one of your patients develops an early cataract. If you believe a patient will need cataract surgery sometime soon, and the patient expresses an interest in the possibility of a multifocal implant, it might not be a bad idea to have him or her try wearing a multifocal contact lens. This will give you some idea about the patient’s ability to tolerate multifocal images, as well as the optical side effects (glare, halos, loss of contrast sensitivity, etc.) It could save you a lot of grief down the road.
Unfortunately, IOL manufacturers don’t make multifocal contact lenses featuring optical technology identical to their multifocal IOLs. If they did, patients could try out the specific visual strategy each lens offers; cataract surgeons could try it as well, to experience what they’re offering to their patients. But even without duplicating the exact visual experience, the current available multifocal contact lenses may provide a clue about whether a patient is a potential multifocal lens implant candidate.
A key part of this strategy is to do this before the patient develops an advanced cataract. Once the patient has an advanced cataract, the media opacity in the lens will make it difficult for the patient to appreciate the benefits of having a multifocal optical system. And, while you’re fitting the patient with contacts, you should also try simulating monovision (using monofocal contacts) to see whether the patient is a candidate for this option. This will also allow you to determine which eye is the dominant distance eye and what the required near add will be for the non-dominant near-vision eye.
The bottom line is that it’s not a good idea to have a patient learn to adapt to a new optical system—be it a multifocal system or a monofocal-monovision system—with cataract surgery lens implants. A contact lens is easily removed; an IOL is not.
Optics Matters
As these examples illustrate, clinical optics plays a key role in just about every decision an ophthalmologist makes. That’s why it’s so important to understand basic optical concepts and principles; doing so can help a clinician enhance the vision—and the lives—of his or her patients.
In a future article, I hope to present more examples of the role of optics in our daily clinical practices as ophthalmologists.
Dr. Korn practices at Sharp Rees-Stealy Medical Group, and is an attending ophthalmologist at Sharp Memorial Hospital in San Diego.