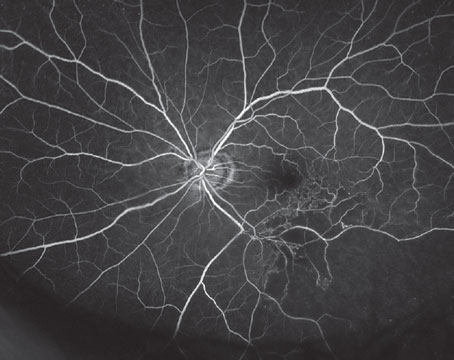
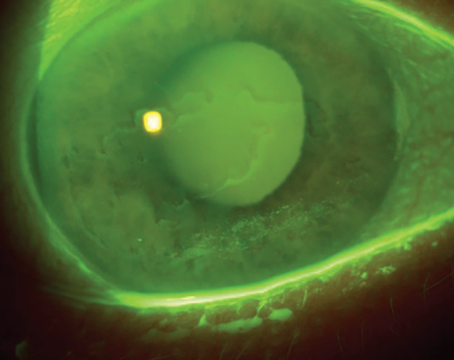
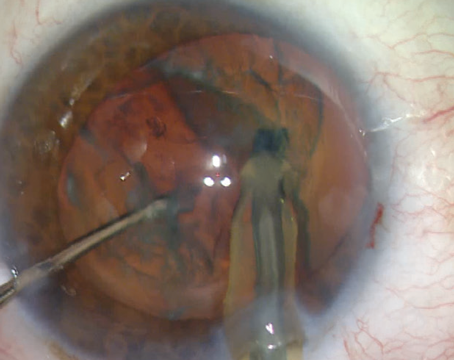
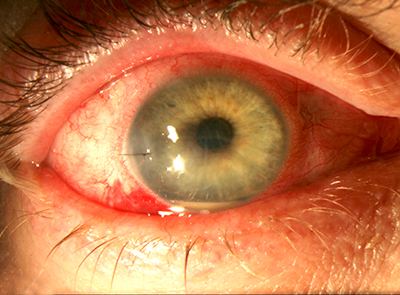
Point: Intracameral vancomycin during cataract surgery is not a good choice for endophthalmitis prevention.
Harry W. Flynn Jr., MD, Anthony D. Anderson, PharmD, BCPS, and Stephen G. Schwartz, MD, MBA, Miami
In 1995 the Centers for Disease Control and Prevention stated that vancomycin should not be used as prophylactic antibiotic; instead, it recommended that vancomycin be reserved for life-threatening or organ-threatening infections.1 Despite that statement, vancomycin has been increasingly used in various specialties of medicine during and after surgery as prophylaxis. This was especially true a few years ago, but since then the use of prophylactic vancomycin has fallen in the United States. The majority of countries are not currently using intracameral vancomycin2 because ophthalmologists recognize the potential complications (TASS, incorrect preparation, cystoid macular edema and contamination).
 |
| Endophthalmitis prevention is one key to successful cataract surgery. Some surgeons believe that the use of intracameral antibiotics adds more risk than benefit. |
Other reasons that intracameral vancomycin is not a good choice for antibiotic prophylaxis include:
• The rates of endophthalmitis without the use of intracameral antibiotics are very low. In fact, many studies have shown that those rates continue to decrease, thanks to the increasing skills of cataract surgeons, mandatory policies and procedures for sterile technique and preparation, and better intraoperative management of complications.3
• Intracameral antibiotics are not a panacea. In our own experience, we’ve seen a number of endophthalmitis cases over the past few years occurring after cataract surgery using intracameral antibiotics. Admittedly, these cases often involved moxifloxacin, which requires preparation by a compounding pharmacy. Also, moxifloxacin provides relatively poor coverage of Staphylococcus.4
• Widespread vancomycin use may contribute to the selection and development of vancomycin-resistant organisms. One of the consequences of the increasing utilization of vancomycin as a prophylactic agent in medicine is that there’s been a worldwide increase in vancomycin-resistant organisms, which in turn has brought about the need for antibiotic stewardship programs in the United States and other countries.5,6 To date, the widespread use of antibiotics in agriculture has been considered the primary source of increased antimicrobial resistance; there are no reported studies regarding the impact of intracameral or topical ophthalmic antibiotics on increased antibiotic resistance. Nevertheless, it’s well known that the MIC-90s of gram-positive organisms have been increasing, impacting our ability to effectively treat gram-positive infections with vancomycin. Meanwhile, our patients may face life-threating infections which require vancomycin. For these reasons, the CDC has pressured hospitals and outpatient surgical centers to monitor and report their antibiotic usage, with added pressure from the Centers for Medicare and Medicaid Services.
• Intracameral vancomycin increases costs to the health-care system. It is estimated that there are approximately 3 million cataract surgeries performed per year in the United States. The exact cost to the health-care system of using intracameral vancomycin is unknown, but those costs go beyond the cost of the drug itself to include the procurement, preparation, storage
• There is currently no FDA-approved, ready-to-use intracameral antibiotic product in the United States. Outside the United States, there is an approved product containing cefuroxime, which has gained popularity in Europe and elsewhere. However, this antibiotic doesn’t cover methicillin-resistant Staphylococcus aureus or methicillin-resistant Staphylococcus
• Intracameral vancomycin can cause hemorrhagic occlusive retinal vasculitis.7,8 The most compelling argument against intracameral antibiotics is the risk of
Most patients count on their cataract surgeon to make the correct decisions regarding their surgery. The entire issue of intracameral antibiotics is controversial, not just regarding vancomycin but with other antibiotics as well. There are significant complications associated with the use of any of these antibiotics. Our patients expect a perfect outcome, and in my opinion, the use of intracameral vancomycin increases the risk of adverse events during or after a procedure that already has a very low rate of endophthalmitis.
Dr. Flynn is the J. Donald M. Gass Distinguished Chair of Ophthalmology at the Bascom Palmer Eye Institute, University of Miami, Miller School of Medicine. Mr. Anderson is
Dr. Flynn and Dr. Anderson have no relevant financial disclosures. Dr. Schwatrz has previously received funding from Alimera and Welch Allyn not directly related to this topic.
1. CDC Bulletin. CDC issues recommendations for preventing
2. Grzybowski A, Schwartz SG, Matsuura K, et al. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns around the world. Curr Pharm Des 2017;23:4:565-573.
3. George NK, Steward MW. The routine use of intracameral antibiotics to prevent endophthalmitis after cataract surgery: How good is the evidence? Ophthalmol Ther 2018 Jul 5
4. Stringham JD, Relhan N, Miller D, Flynn HW Jr., Trends in fluoroquinolone
5. Relhan N, Albini TA, Pathengay A, et al. Endophthalmitis caused by gram-positive organisms with reduced vancomycin susceptibility: Literature review and options for treatment. Br J Ophthalmol 2016;100:446-452. PMID: 26701686.
6. Relhan N, Pathengay A, Schwartz SG, Flynn HW Jr. Emerging worldwide antimicrobial resistance, antibiotic stewardship and alternative intravitreal agents for the treatment of endophthalmitis. Retina, Journal of Retinal and Vitreous Diseases 2017. 37:5: 811-818. DOI: 10.1097/IAE.0000000000001603. PMID: 28338559.
7. Witkin AJ, Chang DF, Jumper JM et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: Clinical characteristics of 36 eyes. Ophthalmology 2017;124:5:583-595. PMID: 28110950.
8.
Counterpoint: Intracameral vancomycin during cataract surgery is a good choice for endophthalmitis prevention.
Richard J. Mackool, MD, Astoria, New York, and Paul Ernest, MD, Jackson, Michigan
The use of an intracameral antibiotic during cataract surgery is now generally recognized as effective in reducing the incidence of postoperative endophthalmitis. Selection of the antibiotic however, requires analysis of both the efficacy of the drug as well as its safety profile. Simply put, we need to know the relative morbidity associated with the use of the current antibiotic options—one of the fourth-generation fluoroquinolones (i.e., moxifloxacin, cefuroxime or vancomycin).
| TABLE I: Intracameral Antibiotics and Post-Cataract Endophthalmitis | |
| Intracameral Antibiotics | Endophthalmitis Incidence |
| none | 1:1,400[1] |
| cefuroxime or fluoroquinolone | 1:5,000[1,2] |
| vancomycin | 0:140,000[3] |
Here is a summary of what we know about intracameral antibiotics and endophthalmitis:
1. If an intracameral antibiotic is not used, the incidence of endophthalmitis is about 1:1,000 eyes;1
2. If either intracameral cefuroxime (ICC) or intracameral moxifloxacin (ICM) is used, the rate of endophthalmitis incidence declines to about 1: 5,000;1,2
3. If intracameral vancomycin (ICV) is used, the incidence of endophthalmitis is astonishingly low. At our ASCs, we now have performed 140,000 consecutive cataract procedures with ICV, and have had no cases of endophthalmitis.3 (Table I, above, summarizes this data.)
There are other factors to be considered in order to objectively compare morbidities with the available ICA options. These include:
• Vancomycin can cause HORV. Approximately 65 percent of eyes that develop HORV will have a final visual acuity of 20/200 or worse.4 At our ASCs, we have identified two eyes with HORV in 140,000 consecutive cataract procedures. We
• Approximately one out of three eyes developing endophthalmitis will have a final visual acuity of 20/200 or worse.1,5,6 The incidence of final visual acuity of 20/200 or worse from endophthalmitis after ICC or ICM is therefore approximately 1:15,000 eyes. (Table II, below, summarizes this data.)
The analysis, however, is a bit more complex. To wit:
1. Approximately 1 percent of patients may be allergic to cefuroxime or fluoroquinolones, and the administration of either drug to these patients may cause TASS. We are aware of a significant number of such cases occurring in a large U.S. cataract practice that began using intracameral moxifloxacin. Patients may not know that they are allergic to these medications, or may report an allergy to a second-generation fluoroquinolone which may be overlooked by medical personnel when planning surgery. Patients who have had an allergic reaction to vancomycin almost always report this when questioned.
2. The most common organism causing endophthalmitis is Staphylococcus, and this organism has demonstrated the ability to develop resistance to previous fluoroquinolone preparations. Because these drugs are commonly administered systemically, it’s likely that the incidence of bacterial resistance to fourth-generation fluoroquinolones will increase.
| TABLE II: Visual Acuity of 20/200 or Worse Following Endophthalmitis after Intracameral Antibiotics | |
| no intracameral antibiotics | 1:3,000 |
| cefuroxime or fluoroquinolone | 1:3,000 |
| vancomycin | 1:93,000 |
(NOTE: The concern that intracameral administration of any antibiotic will promote the development of resistance is unfounded. The latter requires continued antibiotic administration, a microbiologic fact that is widely recognized by experts in that field.)
3. In a similar vein, it’s possible that the incidence of
4. Medicolegal concerns exist as a result of an FDA warning concerning the development of HORV after ICV. However, neither the FDA nor our national organizations (the American Society of Cataract and Refractive Surgery, and the American Academy of Ophthalmology)
5. Bilateral HORV has occurred, and the consequences can obviously be catastrophic. This can be avoided, however, by delaying second-eye surgery for at least two weeks and by performing either a peripheral retinal examination or peripheral retinal photography on the first eye prior to surgery on the second eye.
6. There is some evidence that HORV may be dose-related. In our series of 140,000 patients receiving ICV,
In summary, an objective assessment of the morbidity associated with ICA, using a final visual acuity of 20/200 or worse as our marker, would indicate that the morbidity associated with ICV is significantly less than that associated with ICC or ICM. However, as with all things in medicine—particularly in the area of medications such as antibiotics—the development of increased antibiotic resistance and patient sensitivities must be carefully monitored, and prophylactic ICA administration adjusted accordingly. REVIEW
Dr. Mackool is medical director at The Mackool Eye Institute and Laser Center and senior attending surgeon at the Mt. Sinai New York Eye and Ear Infirmary and New York University Medical Center. Paul H. Ernest, MD, is an innovator, lecturer, author and laser-cataract surgeon at Specialty Eye Institute with 10 offices in Michigan and Ohio. He has performed more than 70,000 cataract surgeries and was the first to use the femtosecond laser for cataract surgery in Michigan. Neither Dr. Mackool nor Dr. Ernest
1. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cat
2. Haripriya A, Chang DF,
3. Mackool R, Ernest P, unpublished data.
4. Witkin AJ, Chang DF et al. Vancomycin associated hemorrhagic occlusive retinal vasculitis. Ophthalmology 2017;124:5:583-595.
5. Al-
6. Pathengay A, Flynn HW Jr, et al. Endophthalmitis outbreaks following cataract surgery: Causative organisms, etiologies, and visual acuity outcomes. Journal Cat