Presentation
A 78-year-old Caucasian male presented to the Wills Eye Emergency Room after seeing “strobe lights” followed by progressively blurred vision in both eyes over a one-week period.
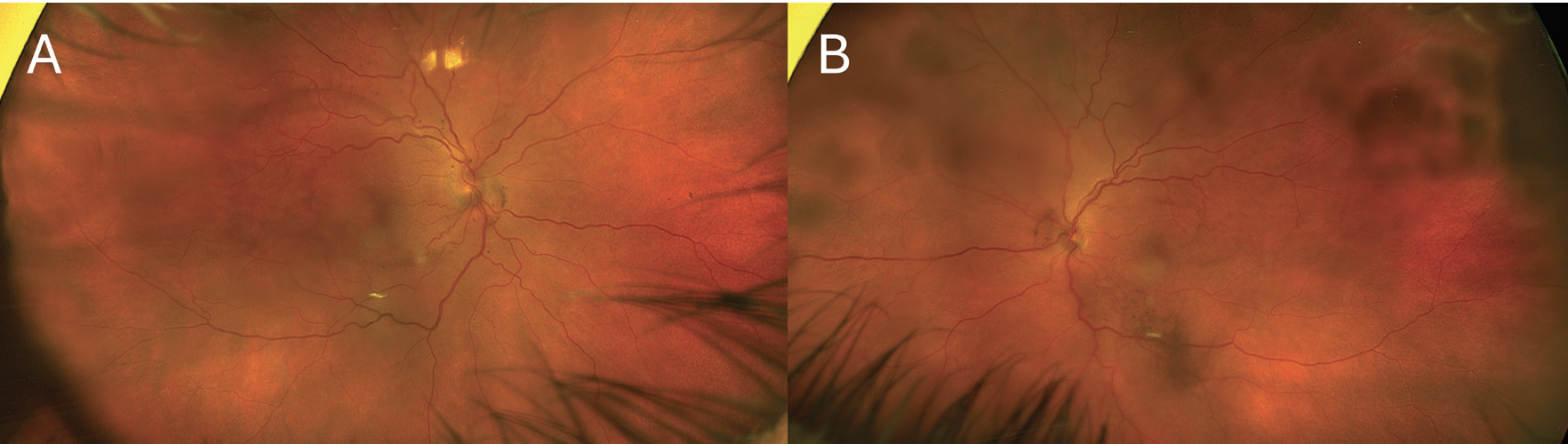 |
| Figure 1. Ultra-widefield fundus photographs of right (A) and left (B) eyes at presentation showing bilateral multilobulated serous retinal detachments. |
History
The patient’s past ocular history was pertinent for a remote history of cataract surgery in both eyes. His medical history was notable for hyperlipidemia, hypothyroidism, gastroesophageal reflux disease, hypertension, spinal stenosis relieved after a lumbar laminectomy, chronic urinary tract infections and bladder incontinence. Previous investigation of his urinary symptoms revealed a high-grade urothelial carcinoma with lymph node involvement diagnosed one year before ophthalmic presentation. His urothelial cancer was treated with a right nephroureterectomy and three cycles of adjuvant chemotherapy with dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC). He was most recently treated with a single dose of maintenance pembrolizumab therapy, just one month prior to presentation.
Review of systems was positive for tinnitus, low back pain and lower extremity edema. He denied trauma, photophobia, ocular pain, vitiligo, headache, scalp tenderness, jaw claudication and unintentional weight loss.
Initial Examination Work-Up
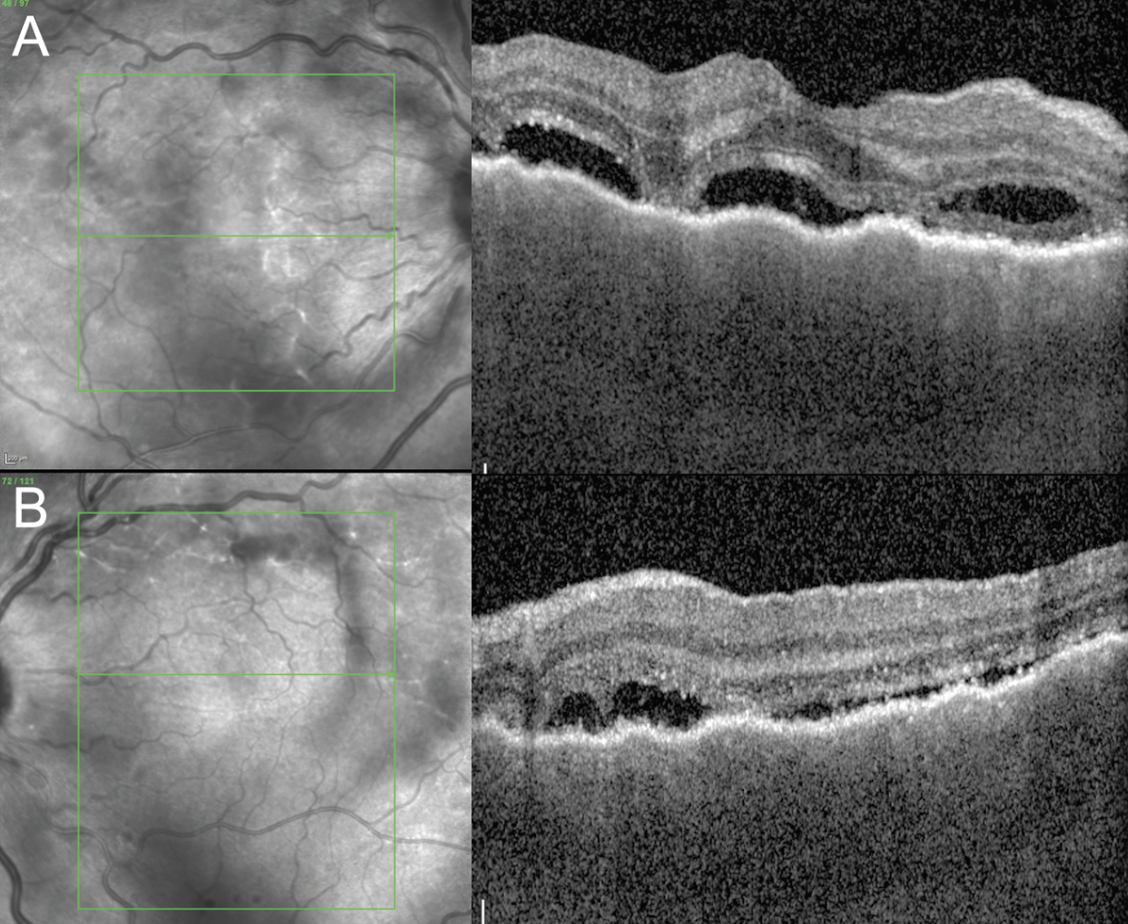 |
| Figure 2. Enhanced-depth imaging optical coherence tomography of the macula of right (A) and left (B) eyes at presentation showing irregularly thickened choroid and subretinal fluid in both eyes and a bacillary detachment in the right eye. |
In the Eye Emergency Room, visual acuity was 20/40 in the right eye and 20/50 in the left. Intraocular pressures were within normal limits. Pupils were reactive with no relative afferent pupillary defect. Ishihara color plates were 6/8 in both eyes. Confrontational visual fields appeared to demonstrate a right homonymous hemianopia. Extraocular motility was full in both eyes without ptosis or proptosis.
Anterior segment examination revealed 2+ anterior chamber cell, a well-centered posterior chamber intraocular lens, and trace anterior vitreous cell OU. Dilated fundus examination revealed a posterior vitreous detachment, multilobulated subretinal fluid in the posterior pole and macular edema OU.
MRI brain and orbits with and without contrast showed mild microangiopathy without any optic nerve pathology, intracranial infarct or mass. Quantiferon gold and Treponemal antibody tests were negative. The patient was started on topical 1% prednisolone acetate six times daily to both eyes and referred to the retina service the next day.
Follow-up examination the next day remained unchanged. Pseudocolor ultra-widefield fundus images demonstrated a hazy media with serous retinal detachments OU (Figure 1). Optical coherence tomography of the macula revealed a thickened choroid and multiple, loculated pockets of subretinal fluid OU and a bacillary layer detachment OD (Figure 2). Fluorescein angiography demonstrated late leakage from the optic nerves, multifocal areas of pinpoint leakage, and pooling in the areas of subretinal fluid in both eyes (Figure 3).
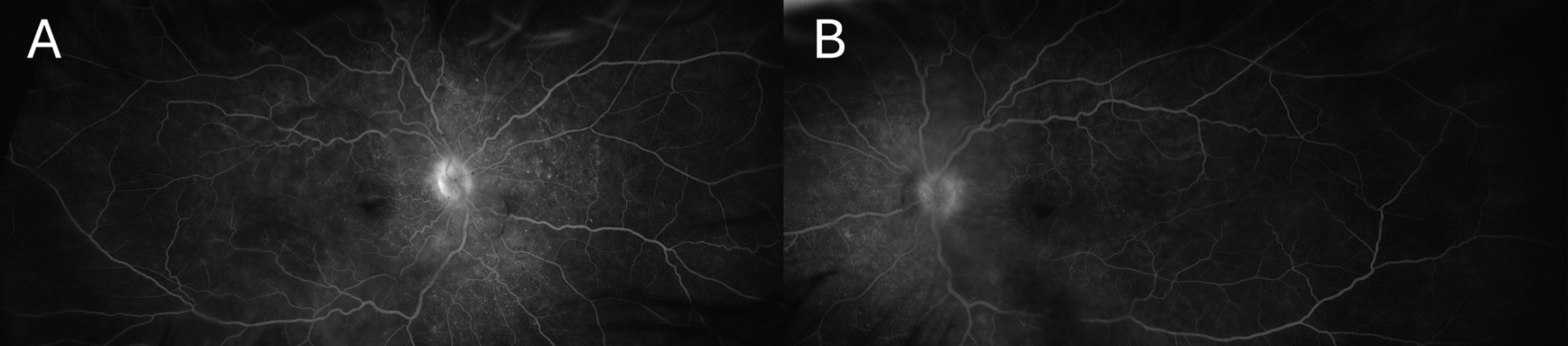 |
| Figure 3. Late phase ultra-widefield fluorescein angiography of the right (A) and left (B) eyes revealing optic nerve leakage, multifocal areas of pinpoint leakage, and pooling in the areas of subretinal fluid in both eyes. |
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Diagnosis and Treatment
We diagnosed this patient with a Vogt-Koyanagi-Harada-like reaction secondary to pembrolizumab therapy for his urothelial carcinoma. Given the important role of pembrolizumab in controlling this patient’s cancer we opted to control the ocular inflammation with local steroids, allowing the patient to continue his cancer immunotherapy without interruption. A 0.7-mg intravitreal dexamethasone implant was placed into each eye. Two weeks later, visual acuity improved to 20/25 OD and remained stable at 20/50 OS. There was notable improvement in the anterior and posterior segment inflammation. On OCT there was resolution of the subretinal fluid and bacillary layer detachment (Figure 4).
Discussion
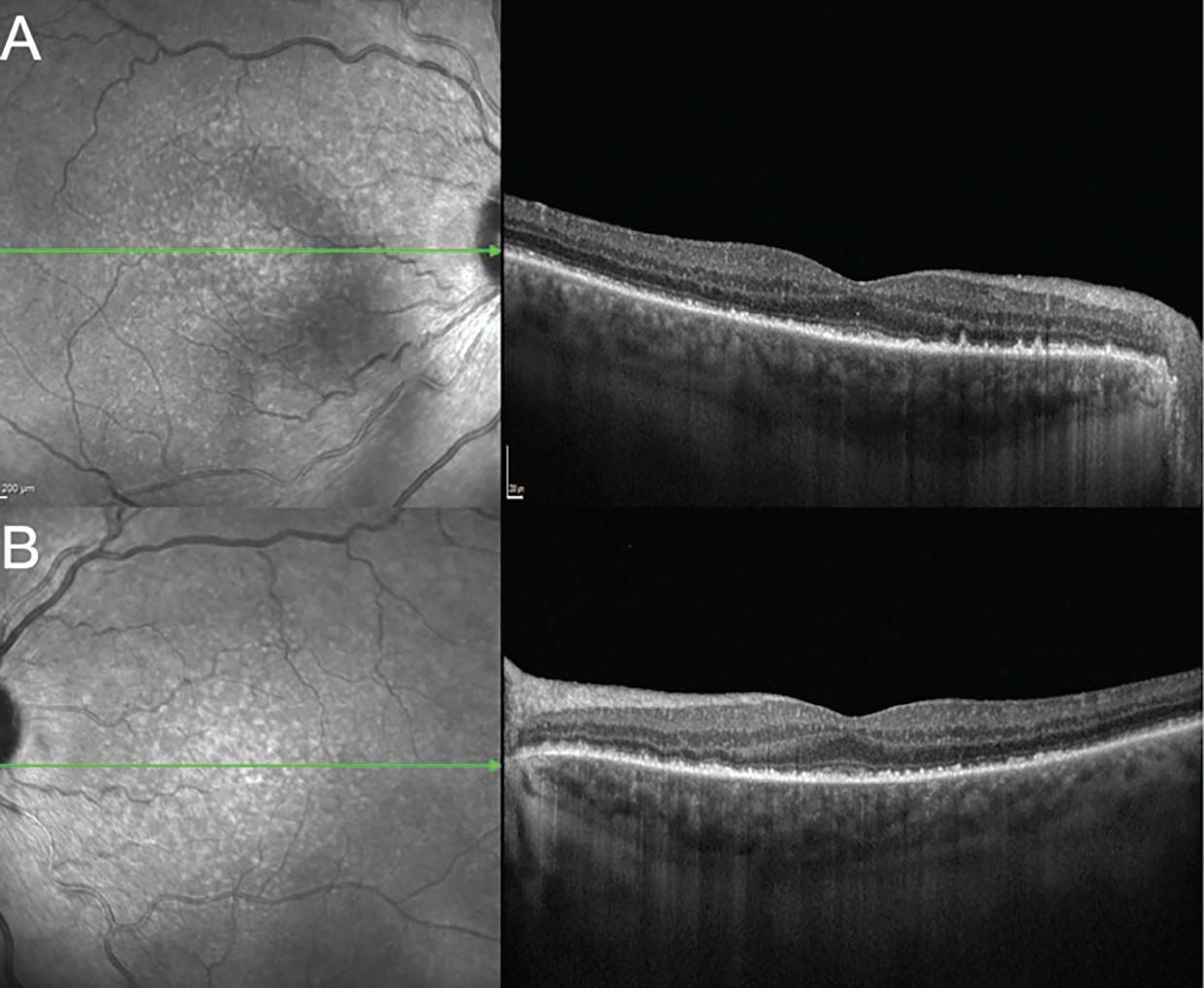 |
| Figure 4. Enhanced depth imaging optical coherence tomography of the macula of right (A) and left (B) eyes showing improvement in choroidal thickening and resolution of the subretinal fluid and bacillary layer detachments after placement of a 0.7-mg dexamethasone implant into each eye. |
We present a case of VKH-like syndrome following treatment of the patient’s urothelial cancer with pembrolizumab which responded well to local steroid therapy, allowing the patient to continue his cancer therapy uninterrupted. A VKH-like syndrome is a known adverse effect of checkpoint inhibitors and has been reported after therapy with ipilimumab, nivolumab, cemiplimab and pembrolizumab.1-4 Notably, upon review of the literature, this is the first case of VKH-like syndrome secondary to pembrolizumab therapy used in the treatment of urothelial cancer. The body has intrinsic mechanisms to prevent autoimmunity, such as inhibitory receptors on T-cells that, when activated, lead to T-cell apoptosis.5,6 Cancer cells can evade the immune system by activating these receptors, which include cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1). Immune checkpoint inhibitors (ICI) are a new class of medications that work by blocking these intrinsic down regulators of the immune system. Pembrolizumab is an ICI and is a monoclonal antibody targeted against PD-1, enhancing the ability of T-cells to detect and eliminate cancer cells. Cancer therapy with ICI has resulted in improved outcomes and survival for many kinds of cancers and are being used more frequently in oncology. However, upregulating the immune system can also lead to inadvertent autoimmunity, which results in immune-related adverse events (irAE).7
IrAEs typically occur within weeks to months of initiating therapy, but can develop at any time, even after cessation. They most commonly involve the skin, gastrointestinal tract, liver and endocrine glands but can occur in any organ, including the eyes.8,9 The incidence and type of irAEs varies greatly depending on the duration and dose of therapy, specific agent used and tumor type. Up to 70 percent of patients treated with a PD-1 or PD-L1 (the ligand that PD-1 binds) inhibitors experience irAEs; however, most of these irAEs are mild to moderate in severity.10,11 Yet, severe and even fatal irAEs have been reported to occur in up to 2 percent of patients.12,13 Fortunately, most irAEs can be successfully managed with corticosteroids.
Ocular side effects from ICIs occur in approximately 1 to 3 percent of patients and most commonly include dry eye, inflammatory uveitis and ocular myasthenia.14-16 There have been relatively few reports of inflammatory orbitopathy, optic neuropathy, retinal vasculitis and VKH-like reactions.17,18 VKH syndrome is an autoimmune condition with ocular, cutaneous and central nervous system manifestations that is believed to result from a T-cell-mediated response to melanocyte antigens.19 A VKH-like syndrome in patients receiving ICIs (as in our patient) is thought to result from T-cell recognition of antigens of noncancerous melanocytes.
Generally, ICI-related uveitis has been shown to respond well to corticosteroids, but the preferred administration route varies based on type and severity of ocular inflammation as well as individual practice patterns. Immunotherapy toxicity is staged using the Common Terminology Criteria for Adverse Events (CTCAE):20 Grades 1 and 2 consist of anterior uveitis with trace and 1-2+ cell, respectively. Grade 3 denotes anterior uveitis with 3+ cell or intermediate, posterior or panuveitis. Grade 4 is reserved for uveitis causing significant vision loss, visual acuity <20/200.
Major oncology guidelines suggest cautious continuation of ICI in Grade 1 and temporary cessation in Grade 2 until inflammation reverts to Grade 1. While most agree with permanent discontinuation of ICI and initiation of systemic corticosteroids in Grade 4 toxicity, the management of Grade 3 events is less clear.21
In a large review of 126 patients with ICI-related uveitis, ICIs were suspended in 11.4 percent and discontinued in 51.4 percent of patients. Topical corticosteroids were the sole treatment in 36.9 percent of cases while 53.2 percent of patients required systemic corticosteroids.22 Yet, in a separate case series of eight patients, all patients (three of which had Grade 3 inflammation) were successfully treated using topical steroids without cessation of immunotherapy.23 As ophthalmologists, having several ways to administer corticosteroids locally, including via a topical, sub-Tenon’s, suprachoroidal or intravitreal approach, may allow for a more nuanced strategy to treat moderate to severe inflammation while avoiding temporary or permanent discontinuation of potentially lifesaving ICIs. We prefer this to systemic corticosteroid treatment given the possibility of systemic immunosuppression interfering with immunotherapy and the increased risk for opportunistic infections.4
In conclusion, we present a case of VKH-like syndrome secondary to pembrolizumab therapy used in the treatment of urothelial cancer with great response to local steroids. This case highlights the importance of taking a thorough history and screening medications in patients with uveitis, especially for medications associated with uveitis like immune checkpoint inhibitors. Additionally, our case highlights the important role of local steroids in these cases, allowing for good control of inflammation and continuation of the patient’s cancer therapy which can be life prolonging or lifesaving. τ
Dr. Deaner has the following disclosures outside of the submitted work: Consultant for Alimera, EyePoint, Regeneron, and Genentech.
Corresponding Author:
Jordan D. Deaner, MD
Mid Atlantic Retina, Wills Eye
Hospital
Assistant Professor of Ophthalmology
Sidney Kimmel Medical College of Thomas Jefferson University
840 Walnut Street, Suite 1020
Philadelphia, PA 19107
jdeaner@midatlanticretina.com
(800) 331-6634
1. Crosson JN, Laird PW, Debiec M, et al. Vogt-Koyanagi-Harada-like syndrome after CTLA-4 inhibition with ipilimumab for metastatic melanoma. J Immunother 2015;38:2:80-4.
2. Kikuchi R, Kawagoe T, Hotta K. Vogt-Koyanagi-Harada disease-like uveitis following nivolumab administration treated with steroid pulse therapy: A case report. BMC Ophthalmol 2020;20:1:252
3. Huang Y, Khan F, Saraiya NV, et al. Vogt-Koyanagi-Harada-like syndrome induced by checkpoint inhibitor cemiplimab. J Immunother 2023;46:8:295-298
4. Bricout M, Petre A, Amini-Adle M, et al. Vogt-Koyanagi-Harada-like syndrome complicating pembrolizumab treatment for metastatic melanoma. J Immunother 2017;40:2:77-82
5. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-42.
6. Jiang, X, Wang, J, Deng, X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019;18:1:10.
7. Puzanov I, Diab A, Abdallah K. et al, Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:1:95.
8. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:2:158-168.
9. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–580.
10. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:26:2455-65.
11. Topalian SL, Hodi FS, Brahmer JR, et al Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:26:2443-54.
12. De Velasco G, Je Y, 0 Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res 2017;5:4:312–318.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:8:711-23.
14. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol 2019;31:3:319-322.
15. Martens A, Schauwvlieghe PP, Madoe A, et al. Ocular adverse events associated with immune checkpoint inhibitors, a scoping review. J Ophthal Inflamm Infect 2023;13:5.
16. Bomze D, Meirson T, Hasan Ali O, et al. Ocular adverse events induced by immune checkpoint inhibitors: A comprehensive pharmacovigilance analysis. Ocul Immunol Inflamm 2022;30:1:191-197.
17. Fortes BH, Liou H, Dalvin LA. Ophthalmic adverse effects of immune checkpoint inhibitors: The Mayo Clinic experience. Br J Ophthalmol 2021;105:9:1263-1271.
18. Dalvin L, Shields C, Orloff M, et al. Checkpoint inhibitor immune therapy: Systemic indications and ophthalmic side effects. Retina 2018;38:6:1063-1078.
19. Rali A, Huang Y, Yeh S. Cancer immunotherapy and uveitis: Balancing anti-tumor immunity and ocular autoimmunity. Int Ophthalmol Clin 2022;62:3:49-63.
20. Common Terminology Criteria for Adverse Events v5.0. Cancer Therapy Evaluation Program. January 6, 2020.
21. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:17:1714-1768.
22. Dow ER , Yung M, Tsui E. Immune checkpoint inhibitor-associated uveitis: Review of treatments and outcomes. Ocular Immunology and Inflammation 2021;29:1:203-211.
23. Parikh RA, Chaon BC, Berkenstock MK. Ocular complications of checkpoint inhibitors and immunotherapeutic agents: A case series. Ocul Immunol Inflamm 2021;29:7-8:1585.



