Iris prosthetics manufacturers include Ophtec BV (Groningen, The Netherlands), maker of the 311 Aniridia Lens II, the Iris Prosthetic System, the Artisan pupil occluder and Artisan Iris Reconstruction IOL; Morcher GmbH (Stuttgart, Germany), maker of black polyethyl methacrylate acrylic (PEMA) devices, including modified capsular
 |
| Morcher aniridia rings are made to be dialed into the capsular bag, one ring on top of the other, so that the occluders interlock. |
Not one of these devices is FDA approved, however. Having served as an investigator for Ophtec BV, Morcher GmbH and HumanOptics AG, Kevin M. Miller, MD, professor of clinical ophthalmology, David Geffen School of Medicine at UCLA, is well positioned to describe the regulatory journey of iris prosthetics in the United States to date. While unrelenting effort is a unifying theme running through endeavors to get devices from all three manufacturers to market, it has not yet paid off. He outlines his experiences with the Ophtec 311 Aniridia Lens, Morcher aniridia implants, and the HumanOptics CustomFlex artificial iris.
Morcher Devices
Morcher makes a variety of black PEMA devices for aniridia, partial iris defects and colobomas. “Internationally, Morcher’s probably the number-one manufacturer,” says Dr. Miller. “Morcher makes two types of aniridia devices: intracapsular tension rings with paddles on them, intended for implantation into the capsular bag, and some sulcus devices. But most of them are meant for the capsular bag. Their other product line is iris-reconstruction lenses. These are combination artificial iris devices and lenses, and the optics come in various diameters,” he says. Dr. Miller’s experience with iris-reconstruction lenses is exclusively with Morcher’s 67B model (a 12.5-mm aniridia implant with a 3-mm optic meant for sulcus placement or scleral suturing). Regarding the company’s modified capsular tension rings, “I have the most experience with the 50F (a 10-mm aniridia ring with occluder panels all the way around, meant to be dialed into the capsular bag against another one) and the 96F (an 11-mm partial aniridia ring with an occluder paddle that covers up to three clock hours), although I have limited experience with some of the others,” he says.
Dr. Miller notes that modified intracapsular tension rings are well suited for dimming colobomas or traumatic iris defects that can be covered by the occluder fins on the devices. “I occasionally get patients sent to me who had cataract surgery, and the iris prolapsed out of the incision and then was pushed back in during the surgery,” he says “Those patients present with significant sectoral iris trauma and transillumination defects where the temporal side of their iris is chewed up and light pours in through that damaged iris as well as going into the pupil. For that particular patient, the ideal implant would be a Morcher 96F device, where you can put the black occluder panel inside the capsular bag. You orient the paddle underneath the damaged iris, and it will block the light coming in through there.” Morcher’s iris reconstruction devices are relatively inexpensive, he notes, but cosmetic outcomes are limited by their lack of color options. “For very dark-colored irises, it doesn’t really matter if you use a black implant because you’re not going to see discrete pupil, per se, but in a blue, blue-green or hazel iris, the option of color is really helpful because the big black defect of those eyes carries with it cosmetic consequences for those patients, in addition to light and glare-sensitivity problems,” he says.
 |
“I’m the only U.S. ophthalmologist that has permission to implant Morcher devices,” Dr. Miller adds. But this is a privilege he had to become a clinical investigator to get, and one he is not exercising at this time: Instead, Dr. Miller wants to bring these devices to the American market so that other surgeons might also use them.
“I actually have my own independent Morcher device trial (Trial Identifier NCT00812708), which I’ve been running for almost 15 years now,” he explains. “In the early 2000’s, I learned how to file for a compassionate-use device exemption. It took a mountain of time and effort to figure it out.” Vowing to never put himself through that process again, Dr. Miller contacted CEO Olaf K. Morcher directly to ask if the company was planning to run a formal device trial in the United States. When Morcher demurred, citing the costs involved, Dr. Miller got his blessing to apply for an IDE and run his own American clinical trial. Dr. Miller and colleagues have investigated compassionate use of several different iris diaphragms,1 the 96F capsular tension ring with a single black occluder panel2 and the 50F iris diaphragm,3 and found them to improve median CDVA and median subjective day and nighttime glare symptom scores.
“Initially, they allowed me to implant 20 devices, and then they allowed me to expand the trial to 70 devices. I used up my allotment several years ago,” he continues. “All I’d have to do is ask the FDA to allow me to implant more, and I’m sure they would, but I want to close this study. If I keep asking for more I’ll constantly be studying these things. It’s hard to
 |
| The CustomFlex artificial iris is made of soft, foldable silicone, so its implantation requires a smaller incision than is needed for rigid artificial irises. |
“Since then I’ve been doing follow-up and data analysis and producing papers on these devices,” he adds. Dr. Miller reports that he’s currently working on his final paper, and focusing his next effort on three papers from his Morcher trial that analyze the devices he implanted most. “My plan is to take that data, after formal analysis, to the FDA and see if I can get these devices on the market under some sort of humanitarian device permission, or maybe even a PMA if they’ll allow me to do that with single-site, single-investigator data,” he says.
CustomFlex Artificial Iris
While Dr. Miller is trying to get Morcher aniridia devices to market, he reports more cause for optimism regarding the CustomFlex artificial iris (HumanOptics AG). “The clinical trial ended a year ago and the regulatory group that’s running the trial out of Cincinnati is preparing to file a PMA application within the next several weeks,” he reports.
The Customflex is the only artificial iris currently available in the United States—albeit on a strictly limited basis. “It’s available for patients who don’t mind entering into a continuing-access clinical trial,” Dr. Miller explains. “The company petitioned the FDA to allow the device to be implanted in patients after the clinical trial, following the same protocol as the trial. There are about 12 investigators from the original clinical trial that are still implanting the device, including me.” The clinical sites are scattered throughout the country, and the protocol requires postop follow-up visits.
At a cost of $5,000 per device, the implant is still out of reach for many patients even without travel-related expenses. “Odds are, there are no investigators anywhere close to you,” says Dr. Miller. “So you’re going to be hopping on a plane for the initial consultation, for the study enrollment visit, the preoperative visit, the surgery and all the subsequent postoperative visits. It’s a big, big hardship.”
While clinical-trial participation is seldom easy for patients or investigators, the CustomFlex has some attributes that make implantation less physically traumatic—an important consideration in these eyes. The CustomFlex’s soft silicon body can be rolled and then laid flat after insertion, allowing for smaller incisions than those required by some Morcher devices.
The CustomFlex can also be trephined to fit individual patients, or cut to cover smaller segmental iris defects. The CustomFlex comes in a standard diameter of 12.2 mm, but as Dr. Miller notes, “Nobody has a really foolproof way of measuring sulcus diameter. A diameter of 12.2 mm fits quite nicely in average eyes. But for the smaller eyes, you need to trephine the device to fit. The most obvious way of doing that is to put the device in and if it looks too big, take it out, trephinate it and stick it back in,” he says. “But implanting these devices is fairly traumatic. You don’t want to place them twice. So most of us that are doing this are sort of guessing as to the sizes we need. I have personally built a trephine set that allows me to cut these devices down in 0.2-mm diameter increments. We don’t really know how to best size these things. There’s room for improvement there.”
Although he thinks the CustomFlex gets a great deal right when it comes to color matching, Dr. Miller also sees room for improvement in that area. The manufacturer sends the surgeon a hand-painted CustomFlex iris, plus a spare, based upon an index photograph of the patient’s fellow eye. They can paint any natural eye color requested in cases of bilateral aniridia. “Some of them turn out to be too light in color: If they’re going to be off, they’ll be off in the too-light direction, rather than too dark,” notes Dr. Miller. “When you place them under the cornea, they appear a couple of shades lighter in the eye, so if you target for exactly the same color as in the photograph of the iris, the implant is going to wind up being several shades lighter than the fellow eye that we photographed. Another thing that makes them ‘pop’ more is that the iris in the injured eye is almost always darker in color than the iris in the normal eye, so we also have a contrast between whatever residual iris is still in the injured eye and the lighter color of the artificial iris. So even if the artificial iris is perfectly matched under the cornea to the color of the good eye, you’re going to see a contrast between the artificial iris and the residual native iris tissue in the eye. There’s no perfect solution.”
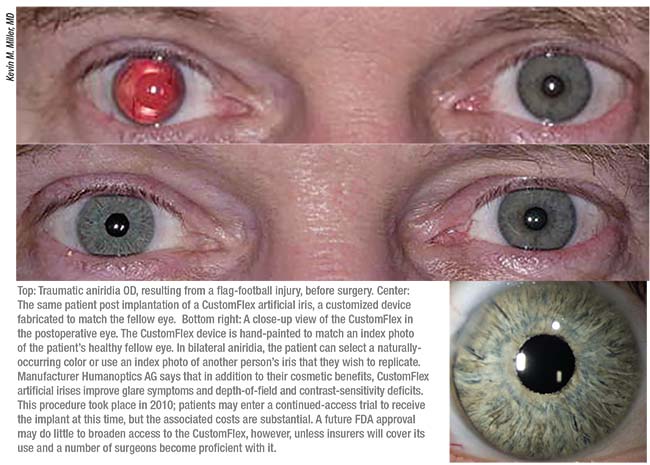 |
Imperfections aside, the lifelike detail of the CustomFlex does more than improve glare sensitivity and photophobia in aniridic patients, according to Dr. Miller. “In the end, except for dark, dark brown irises where you can’t see anything, the cosmetic effects of these devices are actually really important to these patients; the lighter the iris color, the more important they become. The untold story in all of this is that many patients with light-colored irises not only have light and glare sensitivity problems; they also have the emotional baggage that comes along with everybody staring at them, trying to figure out what’s wrong with their eye. They get treated like they’re on drugs or like there’s something else wrong with them. They get told they look weird. Kids stare at them in the grocery store checkout line. These patients carry a lot of emotional trauma from that. They’ve lived with that for a long time,” Dr. Miller says.
Continued Hurdles
Aniridia devices have a difficult road to FDA approval for a number of reasons, including the inherent difficulty of designing studies and quantifying improvements in eyes that tend to have many comorbidities. “These are sick eyes,” notes Dr. Miller. “So we’re taking care of their iris problem in the context of lots of other diseases of the eye. This makes these eyes very hard to study, because the FDA wants to ask, ‘How do they see?’ Well, if they have congenital aniridia, then the best they’re ever going to see is 20/200. But the FDA wants to see a result of 20/20. You can’t get 20/20 out of a 20/200 eye. You can’t get 20/20 out of an eye that’s had three retinal detachment repairs and submacular fibrosis. But those are pretty much average eyes in these studies. So when you compare these device trials to a straightforward lens-implant study where the eyes all end up 20/20, these devices seem terrible. But those are the cards that we’re dealt,” he says.
Efficacy in the CustomFlex trial was measured by reduction of light and glare symptoms via questionnaire responses. Safety was measured by loss of CDVA, by adverse events and secondary surgical interventions (Trial Identifier: NCT01860612).
Prior to the clinical trial, Dr. Miller and colleagues needed to file for compassionate-use exemptions from the FDA to implant the CustomFlex—a process he says requires submitting at least 11 different documents, followed by an IRB application. In early 2017, after the trial for the CustomFlex in the PMA cohort concluded, the manufacturer was allowed to run the continued-access study, which Dr. Miller anticipates will continue for another year or so. “Now all the reports are being generated to file with the FDA to get a formal approval on the clinical trial,” he says.
Eventual FDA approvals will mean little if patients can’t afford the CustomFlex or Morcher devices, Dr. Miller cautions. “Once we get the first device approved, the next problem is going to be getting insurance companies to pay for these things—especially the CustomFlex at $5,000 a pop,” he notes. “You can imagine the pushback we’re going to see from insurers.
“In addition to getting insurance companies to foot the bill for the surgery and the cost of the device, another problem in rolling these devices out is going to be that of training surgeons to use them and avoid all the potential pitfalls,” Dr. Miller continues. “All the surgeons in the clinical trial are really world-class surgeons; they’ve had something of a near-fellowship in the use of these implants. Teaching surgeons how to use the iris devices properly is going to be the final mountain to overcome.”
To that end, Dr. Miller and colleagues offer artificial iris device implantation courses at international meetings for surgeons who want to get ready for the coming technology. “I run one at the ASCRS, Academy, and ESCRS meetings. We get the word out,” he says. REVIEW
Dr. Miller is an investigator for Morcher GmbH and HumanOptics AG, and has served as an investigator for Ophtec BV. He has not received remuneration in any of these roles.
1. Olson MD, Masket S, Miller KM. Interim results of a compassionate-use clinical trial of Morcher iris diaphragm implantation: Report 1. J Cataract Refract Surg 2008;34;10:1674-80.
2. Date RC, Olson MD, Shah M, Masket S, Miller KM. Outcomes of a modified capsular tension ring with a single black occluder paddle for eyes with congenital and acquired iris defects: Report 2. J Cataract Refract Surg 2015;41;9:1934-44.
3. Miller KM, Nicoli CM, Olson MD, Shah M, Masket S. Outcomes of implantation of modified capsule tension rings with multiple black occluder paddles for eyes with congenital and acquired iris defects: Report 3. J Cataract Refract Surg 2016;42;6:870-8.
4. Price MO, Price Jr FW, Chang DF, Kelley K, Olson MD, Miller KM. Ophtec iris reconstruction lens United States clinical trial phase I. Ophthalmology 2004;111:1847-52.



