Retinopathy of prematurity remains the leading cause of blindness in children in the
Unfortunately, the picture is more complicated. ROP screening remains the single highest risk endeavor in the practice of ophthalmology. In the face of high medicolegal risk with accompanying large financial settlements, many trained practitioners are simply unwilling to provide the necessary screening services. Additionally, these services are not well-compensated, often times being reimbursed by Medicaid. The 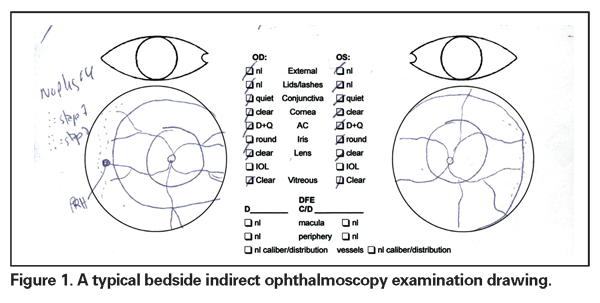
The present gold standard for ROP screening is binocular indirect ophthalmoscopy by an ophthalmologist experienced in the sequential changes of ROP.7 This recommendation comes from the Policy Statement on ROP screening from the AAO, the Section on Ophthalmology from the American Academy of Pediatrics, and the American Association for Pediatric Ophthalmology and Strabismus.7 Interestingly, there has never been a study validating the reproducibility or reliability of this technique.
This is not a diabetic screening examination performed in the comfort of the office. Most ROP screening is initiated in the neonatal intensive care unit (NICU). These are small, premature babies with multiple medical problems and are prone to episodes of apnea, desaturation, bradycardia and tachycardia during the examination. The nurse is often right by the elbow gently encouraging the examiner to "move it along." Oftentimes, the parent is at the bedside or just outside the room. It is in this environment that the bedside binocular indirect ophthalmoscopy examination is performed, following which a drawing is produced for each eye depicting the presence or absence of disease (See Figure 1). Fortunately, there is a common language within the framework of the International Classification of ROP to describe the findings.8,9 Still, I find it troubling that this method has not been rigorously investigated.
We know from studies on fluorescein angiograms that there is wide variability in inter-observer as well as intra-observer interpretation of findings.10-12 Typically, fluorescein angiogram reading is not in the same sort of stressful environment as the NICU ROP examination. Additionally, the fluorescein angiogram has the advantage of not being a moving target—while the observers may change in person or time, each frame of the fluorescein angiogram remains fixed for eternity. This is not the case in ROP screening, where each individual will create the drawing that he sees. These drawings, in my experience, tend to focus on the advancing edge of disease. It seems reasonable that there may be great disparity in the depiction of the status of the fundus from one individual to another in this sort of environment.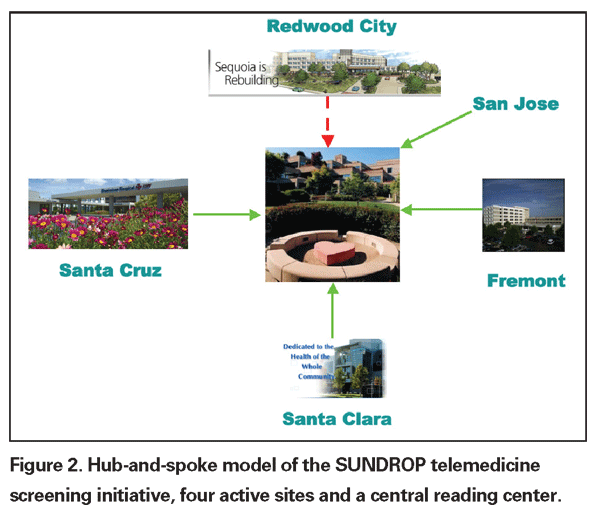
A growing body of evidence has been published on the utility of telemedicine for ROP screening using the RetCam (Clarity Medical Systems,
Four years ago I initiated a series of discussions with the administration of Lucile Packard Children's Hospital at
SUNDROP does not seek to supplant indirect ophthalmoscopy, but rather to streamline the process, identifying the patients with the greatest risk for needing therapeutic intervention. The endpoints are straightforward—identification of referral-warranted ROP (RW-ROP), treatment, or discharge from the NICU. In the SUNDROP network, all babies discharged from the NICU are seen in my outpatient clinic within 72 hours for indirect ophthalmoscopy with scleral depression. Referral-warranted ROP has been classified to include Type 1 or Type 2 ETROP disease, stage 3 disease, plus or pre-plus, and threshold disease.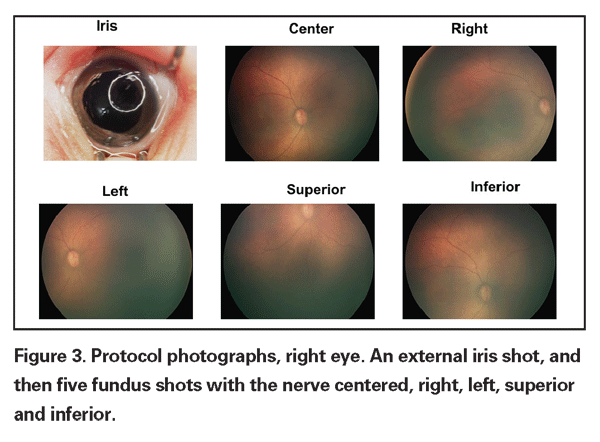
Presently, four sites are enrolled in SUNDROP. Acceptance of the technique and methodology has been high. Results of the first 12 months of the SUNDROP initiative will be presented at this month's AAO meeting in
There is an increasing burden on the practitioners who provide ROP screening services, both from increased eligible infants as well as declining pool of willing screeners. Telemedicine screening for ROP offers the potential to leverage the skills of those few practitioners remaining who wish to continue providing these services.
Dr. Moshfeghi is an assistant professor of ophthalmology in Adult and Pediatric Vitreoretinal Surgery and is co-director of Ocular Oncology at
1. Martin JA,
2. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1988;106:471-9.
3. Multicenter trial of cryotherapy for retinopathy of prematurity. Three-month outcome. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1990;108:195-204.
4. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome—structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1990;108:1408-16.
5. McNamara JA, Tasman W, Vander JF, Brown GC. Diode laser photocoagulation for retinopathy of prematurity. Arch Ophthalmol 1992;110:1714-16.
6. Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology 1993;100:238-44.
7. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006;117:572-6.
8. An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol 1984;102:1130-4.
9. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123:991-999.
10. Friedman SM, Margo CE. Choroidal neovascular membranes: Reproducibility of angiographic interpretation. Am J Ophthalmol 2000;130:839-41.
11. Holz FG, Jorzik J, Schutt F, et al. Agreement among ophthalmologists in evaluating fluorescein angiograms in patients with neovascular age-related macular degeneration for photodynamic therapy eligibility (FLAP-study). Ophthalmology 2003;110:400-5.
12. Kaiser RS, Berger JW, Williams GA, et al. Variability in fluorescein angiography interpretation for photodynamic therapy in age-related macular degeneration. Retina 2002;22:683-90.
13. Lorenz B, Bock M, Muller HM, Massie NA. Telemedicine based screening of infants at risk for retinopathy of prematurity. Stud Health Technol Inform 1999;64:155-63.
14. Schwartz SD, Harrison SA, Ferrone PJ, Trese MT. Telemedical evaluation and management of retinopathy of prematurity using a fiberoptic digital fundus camera. Ophthalmology 2000;107:25-8.
15. Roth DB, Morales D, Feuer WJ, et al. Screening for retinopathy of prematurity employing the retcam 120: sensitivity and specificity. Arch Ophthalmol 2001;119:268-72.
16. Yen KG, Hess D, Burke B, et al. The optimum time to employ telephotoscreening to detect retinopathy of prematurity. Trans Am Ophthalmol Soc 2000;98:145-50; discussion 50-1.
17. Yen KG, Hess D, Burke B, et al. Telephotoscreening to detect retinopathy of prematurity: Preliminary study of the optimum time to employ digital fundus camera imaging to detect ROP. J AAPOS 2002;6:64-70
18. Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity: A pilot study. Ophthalmology 2003;110:2113-7.
19. Mehta M, Adams GG, Bunce C, et al. Pilot study of the systemic effects of three different screening methods used for retinopathy of prematurity. Early Hum Dev 2005;81(4):355-60.
20. Chiang MF, Starren J, Du YE, et al. Remote image based retinopathy of prematurity diagnosis: A receiver operating characteristic analysis of accuracy. Br J Ophthalmol 2006;90:1292-6.
21. Mukherjee AN, Watts P, Al-Madfai H, et al. Impact of retinopathy of prematurity screening examination on cardiorespiratory indices: a comparison of indirect ophthalmoscopy and retcam imaging. Ophthalmology 2006;113:1547-52.
23. Wu C, Petersen RA, VanderVeen DK. RetCam imaging for retinopathy of prematurity screening. J Aapos 2006;10:107-11.
24. Chiang MF, Keenan JD, Starren J, et al. Accuracy and reliability of remote retinopathy of prematurity diagnosis. Arch Ophthalmol 2006;124:322-7.
25. Chiang MF, Jiang L, Gelman R, et al. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol 2007;125:875-80.
26. Balasubramanian M, Capone A, Jr., Hartnett ME, et al. The Photographic Screening for Retinopathy of Prematurity Study (Photo-ROP): study design and baseline characteristics of enrolled patients. Retina 2006;26(7 Suppl):S4-10.



