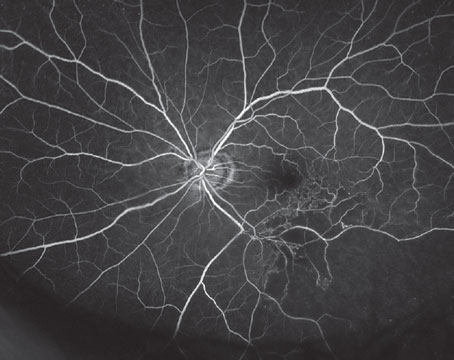
An exponential increase in diabetes has taken place globally in recent decades, with 2021 projections from the International Diabetes Federation estimating that 537 million, or one in 10 adults, aged 20 to 79 live with diabetes. This translates into roughly a billion eyes needing screening for diabetic retinopathy at least once annually, or around three million eyes daily. The amount of images that must be graded in a timely manner has placed an overwhelming burden on human graders, thus leading to more research coming forth about potential aid from artificial intelligence tools.
One new study, published in Ophthalmology Science, prospectively evaluated mydriatic handheld retinal imaging performance assessed by point-of-care AI as compared to retinal imaging graders at a centralized reading center to identify DR and diabetic macular edema.1 A total of 5,585 eyes were observed from 2,793 patients with diabetes.
By reading center evaluation, DR severity in the sample was broken down as:
• no DR—67.3 percent;
• mild nonproliferative DR—9.7 percent;
• moderate nonproliferative DR—8.6 percent;
• severe nonproliferative DR—4.8 percent;
• proliferative DR—3.8 percent; and
• ungradable images—5.8 percent.
DME by reading center evaluation was as follows:
• no DME, 80.4 percent;
• non–center-involving DME, 7.7percent;
• center-involving DME, 4.4 percent; and
• ungradable images—7.5 percent.
Referable DR was present in 25.3 percent of eyes and vision-threatening DR in 17.5 percent. Ungradable images were twice as likely with AI at 15.4 percent; however, there was substantial agreement between AI and the reading center for referrable DR and moderate agreement for vision-threatening DR.
Sensitivity/specificity of AI evaluation was 0.86/0.86 for referrable DR and 0.92/0.80 for vision-threatening DR. Based on these rates, the AI demonstrated it meets the current sensitivity and specificity for referrable DR as set by FDA thresholds of 85 percent and 82.5 percent, but it doesn’t meet the threshold for vision-threatening DR.
Co-author Paolo S. Silva, MD, of the Joslin Diabetes Center’s Beetham Eye Institute in Boston, says one of the motivations behind the study was to make diabetic retinopathy screening more useful for all types of people, rather than just those that were used to make the initial datasets.
“The camera in our study was employed in population that isn’t usually represented in large diabetic retinopathy fundus photo databases,” Dr. Silva says. “The databases generally consist of white, East Asian or South Asian individuals. That doesn’t represent everyone in the world. There’s a vast population that’s underrepresented in these datasets. The end goal for everyone who does diabetic retinopathy screening is to develop a system that will be able to assess everyone, provide care for the remotest populations and in areas that are disadvantaged or have lower resources.”
To this point, the study authors speculate that one potential reason for the high ungradable image rate of the AI is because the algorithm was trained using a different camera type than the one used in the study. A tabletop retinal camera was the source of images used to train the AI, whereas a handheld device was used in the study. While the authors are hopeful this AI technology can be used in the future, they do caution that the high failure rate will need to be addressed in future versions of the software, since this would affect the efficiency and acceptability of AI. To do so, it may need to undergo optimization using a training set of images comparable to the intended population.
“What the study shows is that handheld imaging coupled with AI is a viable option for screening diabetic retinopathy, particularly in remote settings,” says Dr. Silva. “One thing that stood out was the high ungradable rate in this particular study based on the AI we used. But most AI algorithms aren’t trained on handheld retinal images. Handheld retinal images compared to tabletop retinal cameras are very different from each other. Handheld retinal cameras have somewhat low image quality and more imaging artifacts, vs. an office setting, where lighting is optimized and patients may be dark-adapted, and a tabletop camera’s overall image quality is generally better than a handheld retinal camera’s. What you see here—especially if a transition toward community-based screening is going to be done more in the future—is that handheld cameras may be the way to go, and AI algorithms need to be able to adapt to that, by training their algorithms in the future based on datasets from handheld retinal cameras.”
Despite this limitation, the AI has proved efficacy for one of the two main categories, which the authors see as a future solution to the ever-increasing burden of human graders and clinicians, who may struggle to grade all images captured within the day and deal with backlogs as a result. The AI only refers patients at risk of losing their sight for an in-person consult, thus relieving a part of the issue.
As the authors explained in their paper, the use of point-of-care AI and handheld imaging as a DR screening tool “has the potential to decrease the burden on reading centers especially in low-income settings or geographically isolated communities. Reliable AI assessment of DR at point of care with real-time output can guide clinical decision-making and referral recommendations.”
1. Salongcay RP, Aquino LAC, Alog GP, et al. Accuracy of integrated artificial intelligence grading using handheld retinal imaging in a community diabetic eye screening program. Ophthalmol Sci. December 14, 2023. [Epub ahead of print].
Take a Deep Breath ... And Help Prevent Glaucoma?
Spending five minutes taking slow, deep breaths three times per day—a technique called “365 breathing”—is recommended by therapists to help deal with stress, as controlled slow breathing techniques have been shown to shift towards parasympathetic dominance, increase respiratory sinus arrhythmia and augment heart rate variability. As previous studies showing that mindfulness-based stress reduction helps reduce intraocular pressure, researchers recently evaluated the effect of the 365 breathing technique on IOP autonomic functions and stress biomarkers in patients with primary open angle glaucoma and found that it caused a significant reduction in IOP (2 mm Hg) in the intervention group after six weeks of practice.1
A total of 40 subjects in intervention group followed 365 breathing for three times a day at a breathing rate of six cycles per minute for five minutes, in addition to their pharmacological glaucoma treatment. It was explained to subjects that breathing should be smooth, slow, deep and via nasal route, with five seconds devoted to each inhalation and exhalation. “Each day patients were asked to practice first session as soon as they wake up; second session four hours after the first session or just before lunch and the third session at the end of their workday or before starting their evening,” the researchers wrote in their paper.
Another 40 subjects in the control group continued only with their pharmacological glaucoma treatment. IOP, serum cortisol, heart rate variability and heart rate response to a deep breathing test were recorded at pre-intervention and six weeks post-intervention.
The 365 breathing technique caused a significant reduction in IOP (2 mm Hg) and significantly increased the parasympathetic activity in intervention group after six weeks of practice. Previous studies reported a 1.5 mm Hg to 6.1 mm Hg of IOP reduction after a short course (three to six weeks duration) of meditation/mindfulness-based stress reduction in patients with glaucoma/ocular hypertension.
The 365 breathing technique also reduced serum cortisol (stress biomarker) and improved autonomic dysfunction in glaucoma patients.
“Stress is not only a result but also a possible risk factor/cause of glaucoma,” the authors explained. “Acute and chronic stress have been shown to increase IOP. Studies have demonstrated that stress can cause a decline in parasympathetic activity, NO-cGMP dysfunction, endothelial and vascular dysfunction, glial cell activation and downregulation of neurotropins, all of which have been speculated to play a role in complex pathophysiology of glaucoma,” the authors explained.
Cortisol is known to increase in response to stress and alter trabecular meshwork morphology resulting in reduced aqueous humor outflow, thereby elevating IOP, the authors noted.
It’s proposed that the increase in melatonin and nitric oxide could have led to decrease in IOP. An additional decrease in cortisol, as noted in this study, also contributes to decrease in IOP. The decreased sympathetic activity and increased parasympathetic activity can decrease aqueous production and increase aqueous outflow leading to decreased IOP, the authors explained.
“In our study, the IOP reduction after six weeks of practicing 365 breathing was 11 percent and though this is a small reduction, it can help in preventing long-term glaucoma progression. So, the 365 breathing technique cannot be used as a standalone modality to reduce IOP, but can be used as an adjuvant therapy along with glaucoma medications,” the authors explained.
1. Dada T, Gwal RS, Mahalingam K, et al. Effect of ‘365 breathing technique’ on intraocular pressure and autonomic functions in glaucoma patients: a randomised controlled trial. J Glaucoma. Jan 9, 2024 [Epub ahead of print].
Lung Cancer Increases Keratitis Risk by 50 Percent
Epidermal growth factor receptor inhibitors are now some of the most prescribed drugs to treat lung cancer. Ocular side effects have been documented for numerous cancer medications, and EGFRis are no exception; ocular toxicity can manifest as trichomegaly, dry eye or defects of the tear film or epithelium. Adding to this list, a recent study found that EGFRi therapy—especially second-generation afatinib—also increases the risk of new-onset keratitis.
The study involved all patients treated for lung cancer over the last 20 years. Of 1,388,108 total cases identified, 22,225 received EGFRi therapy. The researchers discovered that these patients were 50 percent more likely to develop keratitis than those who didn’t take the drug. Subtypes of EGFRi-associated keratitis included keratoconjunctivitis (hazard ratio, HR: 1.37), superficial keratitis (HR: 1.64) and corneal ulcer (HR: 2.13).
“Notably, keratoconjunctivitis, a common presentation in dry-eye disease, was a frequent subtype observed in this study, suggesting that EGFRi-treated patients may have a higher risk of dry eye,” the researchers wrote in their paper, published in JAMA Ophthalmology.1
Three generations of commonly prescribed EGFRis exist: first-generation gefitinib and erlotinib, second-generation afatinib and third-generation osimertinib. In this study, patients taking afatinib displayed the highest risk of keratitis (HR: 2.23). The authors pointed out that this finding is consistent with past studies that have reported greater adverse effects with afatinib than erlotinib.
There are several mechanisms that could contribute to the elevated risk of keratitis with EGFRi treatment. For one, EGFRi therapy has previously been linked to trichomegaly, “a recognized ocular adverse effect that elevates the risk of corneal cell damage due to abnormal overgrowth and misaligned eyelashes,” the researchers explained. “Additionally,” they continued, “EGFRis inhibit the proliferation, stratification, and migration of limbal and corneal stem cells, impeding the proper repair of damaged corneal epithelial cells.”
When seeing patients undergoing treatment for lung cancer, it’s important to clarify which medication (or medications) they’re taking to assess the potential risk for keratitis. EGFRi-associated ocular effects require prompt diagnosis and management to prevent serious complications or treatment disruptions, the authors urge.
1. Huang P, Lin C, Dana R, Ma KS. Epidermal growth factor receptor inhibitors for lung cancer and the risk of keratitis. JAMA Ophthalmol. January 11, 2024. [Epub ahead of print].