For children with diseased corneas and corneal opacities, the treatment choices have been less than optimal. Performing penetrating keratoplasty in this age group has been fraught with problems, not the least of which is frequent graft rejection due to an active immune system and the need for multiple procedures.
For children with diseased corneas and corneal opacities, the treatment choices have been less than optimal. Performing penetrating keratoplasty in this age group has been fraught with problems, not the least of which is frequent graft rejection due to an active immune system and the need for multiple procedures.
Seven years ago our group at the University of Rochester Flaum Eye Institute attempted a new approach: using the Boston I keratoprosthesis in these youngest of patients. Since then we’ve implanted the device in 60 pediatric eyes, almost all in children under age 5 and about half in newborns and infants younger than 12 months. While the nature of the pathology and the potential for complications are ever present, the visual and eye health outcomes in these patients have been markedly better than what we would have expected with PK.
In this article I’ll review the considerations, indications, perioperative management and potential complications of using the Boston I keratoprosthesis, or Kpro. This polymethylmethacrylate-composed artificial cornea was developed by Harvard ophthalmologist Claes Dohlman and has been available commercially since 1992, but its present modification only since 2002. A host of studies have validated Kpro as an effective treatment for corneal disease in adults.
Why Kpro for Kids?
Our total series with the Kpro is approaching 400 adult and children’s eyes. Because of advances in the device’s design as well as surgical technique, postoperative care and management, the paradigm for using the keratoprosthesis shifted.1 Where it was once considered a last resort after multiple failed graft attempts, we now embrace the Kpro for a much wider series of adults, sometimes as the primary procedure, and often in patients with diseased corneas but otherwise normal eyes.
| Because of advances in the Kpro's design as well as surgical technique, postoperative care and management, the paradigm for using the keratoprosthesis shifted. |
The literature on PK in infants has not been compelling.2 A 2008 series of 40 procedures in 32 patients showed a 22-percent graft survival at two years.3 Another series of 144 PK procedures in 72 eyes and 47 patients a year later showed only 29 percent had 20/400 visual acuity or better at minimum three-year follow-up.4 Our results with the Kpro compare favorably to these series, and only two of 60 implants resulted in extrusion.
 |
With the Kpro the optics are stable and from the first day capable of transmitting a clear image to the retina. Evaluation of the quality and clarity of the graft is difficult in infants because one cannot use the traditional slit lamp in such small patients and examination is only possible under anesthesia. Because these cases are rare and the prime cause of congenital opacity (Peters’ anomaly) has a variety of expressions, each case is unique.
Another option for corneal opacity in the infant is an optical iridectomy, but this procedure, while safer, has its limits—mainly that it’s indicated for an opacity that does not involve the entire cornea and only when the eye is otherwise disease-free. The infants we’re dealing with often have iris aberrations, cataract, anterior and posterior synechiae, vitreous and retina changes and glaucoma along with the corneal disease.
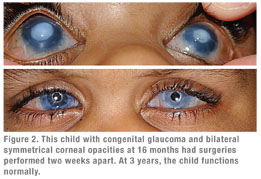 |
Treating corneal opacities, especially bilateral cases, is vital to a child’s development. Because vision is a learned process, getting a clear image to the back of a child’s eye as early as possible is critical for training the brain to process visual images. That means getting 20/400 or better VA into one eye so the child can develop normally. We also believe in treating unilateral opacities as well, even though this approach can be more controversial outside the United States. In many places in the world, there is a reluctance to expend resources to rehabilitate one eye when the fellow healthy eye may be sufficient for a normal life. A problem with this concept is that it is difficult to predict normality in newborns. Astigmatism, high refractive error, macular disease and glaucoma may not be detected until the opportunity for preventing amblyopia in the poorer eye has passed.
But every day the retina is deprived of a clear image is a day that contributes to the development of amblyopia. The question is, how soon should you treat the eye? No one is quite sure, and the answer can vary from patient to patient depending on brain plasticity. Some brains are less prone to amblyopia than others, but the pediatric ophthalmologist has no way of knowing that. With congenital cataract, which is far more common than corneal opacity or Peters’ anomaly, the operation is routinely done at age six weeks to 14 weeks. Our approach is to operate on the eye as early as possible.
Timing is important, but preoperatively there are several steps that can consume precious time. Getting a patient history is not always a simple process. Our process often involves several consultants. We also like to perform the early evaluation under anesthesia. This evaluation includes intraocular pressure, A-scan ultrasound to determine the eye’s axial length, a B-scan to evaluate the retina, perhaps visual evoked response to assess the eye’s reaction to light, and maybe a brain scan in the setting of other pathology to evaluate potential brain function.
Another reality is dealing with insurance. Prior authorization can take up to two weeks. One of the largest private insurers has only recently allowed ready access to the surgery in infants. Cases with concurrent pediatric disease need to have a pediatrician involved. If the newborn is in a neonatal intensive care unit, we may have to arrange for a transfer to another NICU. For the procedure itself, we need to schedule a pediatric anesthesiologist. Then we have to determine what power of the Kpro device to use and, if it’s not in inventory, order one. The device itself can cost $5,000 and additional costs involve the cornea tissue necessary for implantation, operating room time, anesthesia, etc.
We also need to schedule the OR time and coordinate with other members of the care team (glaucoma and retinal specialists, pediatric ophthalmologist). All of this needs to be coordinated with the parents’ schedule. Since most of the cases are referred from quite a distance, communication and transportation are issues. Sometimes as soon as possible may mean two or three months rather than two or three weeks.
Considerations for Kpro in Kids
Indications for Kpro in children fall into two categories:
• congenital, including Peters anomaly, sclera, cornea, glaucoma causing corneal scarring, congenital hereditary endothelial dystrophy (CHED) and nondescript dysgenesis of the eye; and
• non-congenital, such as forceps injury, birth canal infection and trauma.
For CHED, replacing just the endothelial cells in the cornea may be a safer procedure than the Kpro implant, but since the condition is quite rare that approach is only appropriate in perhaps less than 5 percent of all infant cases. Our series of 60 eyes included only one patient with CHED.
Operating on these patients can be challenging for many reasons. These are not merely small eyes. Patients with Peters’ anomaly often have a host of other medical problems, both ocular and otherwise. Patients with Peters I can have cataract, glaucoma, retinal disease and vitreal inflammation.5 Peters I has a strong association with myopia, aniridia, coloboma, synechiae, persistent hyperplastic primary vitreous, optic disk hypoplasia and systemic anomalies.6
Peters II is associated with systemic problems such as craniofacial abnormalities, cardiovascular disease that can require coronary artery bypass grafting and heart valve replacement, or gastrointestinal problems requiring intestinal surgeries.
An additional consideration is that almost half of our patients present with multiple prior surgeries, including failed grafts, adhesions, cataract and glaucoma, requiring more complicated surgical management.
 |
 |
The process of implanting a keratoprosthesis in an infant is radically different from performing PK. The workup and ocular evaluation are similar; they require anesthesia because infants cannot cooperate and respond to the examination the way an older child can. The similarities end there. When I did PK in these patients, I operated alone or with members of our cornea team. That’s not the case with implanting a prosthetic cornea.
Kpro in children demands a multidisciplinary approach. Besides the corneal surgeon, a pediatric glaucoma specialist, a vitreoretinal specialist and the pediatric ophthalmologists for long-term amblyopia therapy are all team members. Scarring of the cornea, for example, tends to change its tensile strength and flaccidity, hence the need for the pediatric glaucoma specialist to determine the presence of the condition and put into place appropriate therapy. On occasion the elevation in pressure requires initial glaucoma intervention.
Our procedure has evolved to go beyond just replacing the cornea. After I insert the prosthesis, the vitreoretinal specialist routinely performs a total pars plana vitrectomy combined with laser photocoagulation of any retinal damage. We adopted this approach based on our earlier experience in binocular cases of performing monocular vitrectomy. We found the non-vitrectomy eyes had a higher incidence of vitreal inflammation that can lead to a secondary retinal detachment.
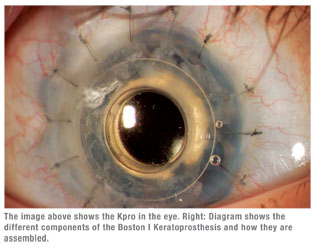
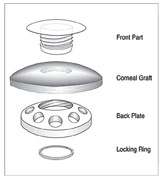
We implant the Kpro with a ring of donor cornea. That helps the device become incorporated into the eye, eliminating any distortion as the eye grows and the cornea grows around the prosthesis.
Postoperative Management
After the operation, we place a bandage contact lens over the prosthesis. This is important in preventing or delaying ocular surface problems and helps prevent corneal melt. We prescribe topical steroids typically q.i.d., but six to eight times a day when inflammation is present. These eyes are highly prone to inflammation. We prescribe antimicrobial drops once or twice a day. Of course, with steroid use as well as inherent potential for the disease, the eyes must be monitored closely for glaucoma.
The care team must stay involved as well. The vitreoretinal specialist needs to evaluate the eye with ultrasound at three months postoperatively and then every six months—again, under anesthesia until the child is old enough to cooperate. The pediatric ophthalmologist likewise has to follow up every six months to monitor anti-amblyopia therapy. Also as the child grows, so does the eyeball, requiring frequent changes in vision correction.
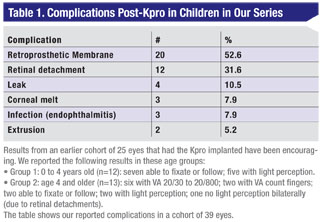
While Kpro avoids rejection and is comfortable for the child, eyes with Peters’ anomaly or other forms of inflammatory disease are more prone to complications than other eyes. Postoperative complications can include bandage contact lens management, retroprosthetic membrane vitreoretinal disease, endophthalmitis, melting or thinning, extrusion and glaucoma.7
Long-term, the Kpro provides a baseline for correcting VA in the future. As the child grows, so does the axial length of the eye. We calculate in choosing the keratoprosthesis and then change the power of the contact lens over the prosthesis accordingly. At age 5 or 6, the child can begin wearing glasses as an alternative.
PK and patching to treat corneal diseases and opacities in infants and other children under age 5 can be fraught with failure. We’ve found that using a keratoprosthesis, while more invasive and resource-dependent, can resolve many of these problems and is our procedure of choice in dealing with congenital corneal opacity.
Dr. Aquavella, the first fellowship- trained corneal surgeon in the United States, is a professor of ophthalmology at the University of Rochester School of Medicine and Dentistry Flaum Eye Institute in Rochester, N.Y. He has been involved in keratoprosthesis surgery and research for 40 years.
1. Aquavella JV, Qian Y, McCormick GJ, Palakuru JR. Keratoprosthesis: The Dohlman-Doane device. Am J Ophthalmol 2005;140:1032-1038.
2. Aquavella JV, Gearinger MD, Akpek EK, McCormick GJ. Pediatric keratoprosthesis. Ophthalmology 2007;114:989-94.
3. Rao KV, Fernandes M, Gangopadhyay N, Vemuganti GK, Krishnaiah S, Sangwan VS. Outcome of penetrating keratoplasty for Peters anomaly. Cornea 2008;7:749-753.
4. Yang LL, Lambert SR, Drews-Botsch C, Stulting RD. Long-term visual outcome of penetrating keratoplasty in infants and children with Peters anomaly. J AAPOS 2009;13:175-180.
5. Peters A. Ueber angeborene Defektbildung der Descemetschen Membran. Klin Mbl Augenheilk 1906;44:27-40, 105-119
6. Althaus C. Sundmacher R. Keratoplasty in newbornes with Peters’ anomaly. Ger J Ophthalmol 1996;5:31-35.
7. Chak G, Aquavella, JV. A safe Nd:Yag retroprosthetic membrane removal technique for keratoprosthesis. Cornea 2010 Epub in press.