In this article, we aim to provide a simple, five-step approach to increasing your success rate, which we define as clear corneas on postop day one for most of our patients.
The Challenge of PBK
The main cause of PBK is complicated cataract surgery with inadequate management of posterior capsular rupture or intraocular lenses placed in the anterior chamber. PBK can occur in 1 to 2 percent of patients after conventional cataract surgery, although this incidence can increase to 11 percent2 and up to 24 percent3 in cases with an endothelial cell count below 1,000 cells/mm2. In about 5 percent of patients in need of cataract surgery, the surgeon will be able to identify one or more risk factors for PBK, including Fuchs’ endothelial dystrophy, a shallow anterior chamber, angle closure glaucoma, previous eye surgery and/or very dense cataracts. Following is the systematic approach we take.
1. Recognize Eyes at Risk
Every eye undergoing cataract surgery is different, so the first step for success is to recognize eyes that are at risk for PBK. The following factors should be considered when assessing the risk of endothelial failure:
• Advanced age. Endothelial cell loss is known to occur physiologically over time.
• Systemic conditions. Conditions such as diabetes mellitus,4 renal insufficiency5 and chronic occlusive pulmonary disease6 are known to decrease endothelial cell count and function.
• Medications. Drugs such as amantadine for Parkinson’s disease or topical carbonic anhydrase inhibitors such as dorzolamide can affect corneal endothelial function. Also, medications such as tamsulosin increase the risk of posterior capsular rupture.
• Classification of nuclear cataract. Dense nuclear cataracts require more ultrasound energy and prolonged surgical time, are more prone to turbulence and chatter during phacoemulsification, and are technically more
difficult to operate on.7
• Fuchs’ endothelial dystrophy severity and other features. FED severity can be classified using the Krachmer scale: Mild FED includes patients with nonconfluent guttata; moderate FED has confluent guttata in a diameter between 1 and 5 mm; and severe FED has confluent guttata in an area larger than 5 mm, with or without corneal edema.
 |
Moderate and severe FED cases have significantly decreased endothelial cell counts and, in some instances, increased corneal thickness.8 Endothelial cell counts in patients with FED are often not reliable per se; and the surgeon must check the amount of guttata in the image to determine the quality of the endothelium, taking into consideration the coefficient of variability, hexagonality (polymorphism and polymegathism)9 and the ultrasound pachymetry. Traditionally, a pachymetry greater than 600 µm indicated the need for a triple procedure (combined phacoemulsification, IOL implantation and endothelial transplantation), but this is shifting towards a pachymetry of 630 to
640 µm based on more recent research.10 A newer method, the measurement of epithelial corneal backscatter, seems promising, but it’s still not clinically available to most cataract surgeons.11
With current diagnostic methods, we lack a specific number in terms of endothelial cell count or pachymetry to determine if a FED-affected cornea will be able to withstand cataract surgery and remain transparent or if it will require a triple procedure.12 Patients with advanced FED manifest clinically with blurred vision in the morning, which then improves as the day progresses. These symptoms indicate endothelial dysfunction and are extremely important to elicit during your preoperative evaluation.
• Previous eye surgery. Previous ocular surgery such as penetrating keratoplasty, peripheral iridotomy, trabeculectomy, intraocular lens implantation, glaucoma valve implantation or previous retinal surgery with the use of silicone oil can decrease the endothelial cell count and should be considered risk factors for PBK.
• Small eyes. Eyes with a very shallow anterior chamber, short axial length and primary angle closure glaucoma tend to have lower endothelial cell counts. Intraoperatively, the distance between the cornea and the phacoemulsification tip is naturally reduced in these eyes, which implies a higher amount of ultrasound energy indirectly delivered to the endothelium.
2. Score the Risk
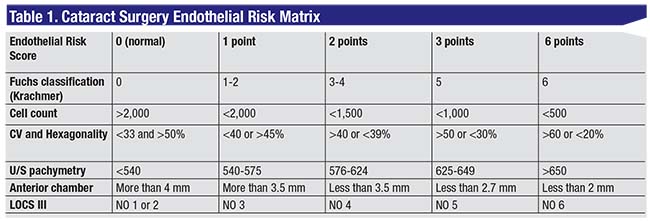
The second step for success is to score the level of risk for the eye. In order to evaluate the risk, we evaluate:
• the quality of the endothelium on the Krachmer scale for FED, endothelial cell count and ultrasound pachymetry;
• anterior chamber depth by conventional biometry or other methods; and
• the degree of cataract. We use the LOCS III classification, but another method can be substituted according to your preference.
Variables evaluated in the risk matrix (appearing in Table 1) are the severity of FED, endothelial cell count, coefficient of variability, hexagonality, ultrasound pachymetry, anterior chamber depth and LOCS III cataract grade classification. Interpretation of the risk score follows in step 3.
3. Customize the Surgery
The third step for success is to be aware of your surgical options and use them according to the score calculated in step 2:
• Low risk (0 to 5). In low-risk patients, such as those with soft cataracts, normal ACD and few guttata with no other corneal abnormalities, a normal phacoemulsification procedure with controlled fluidics can be performed with very low risk.
• Moderate risk (6 to 10). An example of a moderate-risk case would be someone with moderate FED, with a cell count above 1,500 cells/mm2 and a cataract LOCS III grade 4. In such a patient, prechopping, ultrasound-sparing techniques (femtosecond laser-assisted, Akahoshi prechop, ultrachop,13 etc.) should be used. Also, dispersive OVD injection should be repeated every three to five units of effective phacoemulsification time during quadrant removal.
• High risk (Score 11 to 19). Zero-ultrasound techniques such as extracapsular cataract extraction or manual small incision cataract surgery should be considered in these cases, paying special attention to avoiding contact between the nucleus and the endothelium, as endothelial contact can be just as damaging to the compromised endothelium as a normal phaco.
Viscodynamic extraction is another zero-phaco technique that can be used. It involves a sclero-corneal tunnel, small fragmentation of the nucleus using any method (femtosecond laser,14 ultrachopper15 or Akahoshi prechopper), and subsequent fragment removal through the sclerocorneal wound while dispersive viscoelastic is injected liberally into the anterior chamber to push the fragments out of the eye.
• Very high risk (20+). In these patients, the surgeon should consider a triple procedure with Descemet’s stripping endothelial keratoplasty, ultrathin Descemet’s stripping automated endothelial keratoplasty or Descemet’s membrane endothelial keratoplasty, according to his/her preference.
4. Avoid Intraoperative Damage
Adhere to the three pillars of endothelial protection: Use minimum or zero ultrasound energy near the corneal endothelium; maintain a stable, closed fluidics system; and keep a normal-to-low IOP.
Increasing the distance from the cornea to the tip of the phaco probe reduces the amount of effective U/S energy at the level of the endothelium. Working on the iris plane, as far as possible from the cornea, reduces the likelihood of corneal damage. Holding the U/S tip downwards, away from the endothelium, can also avoid the direct effects of U/S energy.16 Similarly, placing dispersive viscoelastic for every three to five units of cumulative dissipated energy against the endothelium creates a barrier between the U/S tip and the cornea.17,18
Working in a closed system (i.e., avoiding fluid loss through the paracentesis) prevents fragments from contacting the endothelium and allows for a deeper AC. Slow-motion parameters reduce turbulence and chatter, help reduce the risk of fragments hitting the endothelium19 and help avoid posterior capsular rupture, which is a major risk factor for PBK.
We routinely favor the use of preop IV mannitol or oral acetazolamide if the anterior chamber is less than 2.8 mm deep or there is history of glaucoma. Higher IOPs can induce more corneal endothelial damage and low IOPs can result in anterior chamber shallowing with undesired consequences.
5. Discuss the Risks
Have a conversation with the patient about the risks and possible complications of the case. Patients have the right to understand their problem as well as their alternatives. Regional differences and the availability of corneal tissue play a significant role in the surgeon’s decision. Similarly, each surgeon should be aware of his own level of expertise both with cataract surgery and endothelial transplant techniques in order to decide the best option for the patient at any given time.
Performing early surgery can be best in soft cataracts, while waiting until the cataract has caused significant visual loss can be the best option in high-risk cases when corneal tissue isn’t readily available. In advanced FED cases with moderate cataracts, it can be acceptable to perform an U/S-sparing cataract surgery technique to reduce the possibility of corneal decompensation, but the patient should be aware of the possibility of endothelial corneal transplantation in the near future.
We hope that this discussion of the major risk factors and our simple tool for risk stratification of corneal endothelial damage in the setting of cataract surgery, has been helpful. The selection of surgical technique will depend on the surgeon’s preference and expertise, and the three pillars of endothelial protection provide a simple guide every time we decide to perform phaco. By following this approach, the surgeon will be more likely to obtain clear corneas on the first postoperative day for most cases, and we’ll all help conserve a precious and limited resource: corneal tissue.
The authors practice at Clinica Oftalmológica del Caribe in Barranquilla.
1. Pineda R. Corneal transplantation in the developing world: Lessons learned and meeting the challenge. Cornea 2015;34 (Suppl):S35-S40.
2. Yamazoe K, Yamaguchi T, Hotta K, et al. Outcomes of cataract surgery in eyes with a low corneal endothelial cell density. J Cataract Refract Surg 2011;37:2130-2136.
3. Hayashi K, Yoshida M, Manabe S, Hirata A. Cataract surgery in eyes with low corneal endothelial cell density. J Cataract Refract Surg 2011;37:1419-1425.
4. Shenoy R, Khandekar R, Bialasiewicz A, et al. Corneal endothelium in patients with diabetes mellitus: A historical cohort study. Eur J Ophthalmol 2009;19:369-375.
5. Diaz-Couchoud P, Bordas FD, Garcia JR, et al. Corneal disease in patients with chronic renal insufficiency undergoing hemodyalisis. Cornea 2001, 20:695-702
6. Soler N, Romero-Aroca P, Gris O, et al. Corneal endothelial changes in patients with chronic obstructive pulmonary disease and corneal vulnerability to cataract surgery. J Cataract Refract Surg 2015;41:313-319.
7. Ishikawa A. Risk factors for reduced corneal endothelial cell density before cataract surgery. J Cataract Refract Surg 2002;28:11:1982-92.
8. Kopplin LJ, Przepyszny K, Schmotzer B, et al. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch ophthalmol 2012:130:433.
9. Rao GN, Aquavella JV, Goldberg SH, Berk SL. Pseudophakic bullous keratopathy; relationship to preoperative endothelial status. Ophthalmology 1984;91:1135-1140.
10. Seitzman GD. Cataract Surgery in patients with Fuchs Corneal Dystrophy. Ophthalmology 2005;112:441-446.
11. Cleynenbreugel HV, Remeijer L, Hillenaar T. Cataract surgery in patients with Fuchs´ endothelial corneal dystrophy: When to consider a triple procedure. Ophthalmology 2014;121:445-453.
12. Kohnen T. Compromised corneal endothelium and cataract: How should we decide? J Cataract Refract Surg. 2011;37:8:1377.
13. Galvis V, Tello A, Escaf LJ, Rojas V, Cortez MA. Phaco prechopping as an option in high-volume cataract services. Tech Ophthalmol 2007;5:1:1-7.
14. Conrad-Hengerer I, Al Juburi M, Schultz T, et al. Corneal endothelial cell loss and corneal thickness in conventional compared with femtosecond laser-assisted cataract surgery: Three-month follow-up. J Cataract Refract Surg 2013:39:1307.
15. Crandall AS. Ultrasonic pre-chopper. Available at: http://ascrs2011.conferencefilms.com.
16. Faramarzi A, et al. Corneal endothelial cell loss during phacoemulsification: Bevel-up versus bevel-down phaco tip. J Cataract Refract Surg 2011;37:1971–1976
17. Arshinoff SA. Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg 1999;25:2:167-73.
18. Tarnawska D, Wylegata E. Effectiveness of the soft-shell technique in patients with Fuchs’ endothelial dystrophy. J cataract Refract Surg 2007;33:1907-1912.
19. Schriefl SM, Stifter E, Menapace R. Impact of low versus high fludic settings on the efficacy and safety of phacoemulsification. J Cataract Refract Surg 2011;37:12:2130-6.



