A sclero-keratoplasty is a surgical procedure that can be used to preserve the eye in the setting of bacterial keratitis with scleral extension. In cases of severe scleromalacia in the setting of infectious keratitis, large donor grafts may be used to excise persistent keratitis and preserve ocular function. In cases of severe bacterial keratitis, a tarso-conjunctival flap may help aid in scleral coverage, by providing conjunctiva and a blood supply to assist with surface repair and healing.
Here, we describe two cases in which a tarso-conjunctival flap is used in conjunction with a sclero-keratoplasty to treat scleromalacia in the setting of bacterial keratitis and in the setting of endophthalmitis.
 |
Infections and Sclero-keratoplasty
Bacterial keratitis, an acute or chronic infection of the cornea, has a reported incidence of 28 per 100,000 in the United States and an increased incidence of 130 per 100,000 among contact lens wearers.5 Though the leading cause of bacterial keratitis is prolonged use of contact lenses, possible underlying factors consist of ocular surface diseases, corneal trauma, use of immunosuppressive medications and postocular surgery.2 Topical antibiotics are the mainstay of treatment with consideration of systemic antibiotics in severe infections. In cases of corneal thinning or perforation, the physician can attempt corneal gluing.3 For deeper perforations, a small or large diameter patch graft may be used based on the size, depth and location of the ulcer. In instances involving scleromalacia, sclero-keratoplasty has shown to be a successful surgical intervention for patients.
While the use of sclero-keratoplasty has proven to be an effective method in repairing corneal defects, it’s been associated with rejection and, most notably, post-keratoplasty infectious keratitis. PKIK typically involves infection stemming from gram positive bacteria and fungi such as Candida spp. Risk factors include topical corticosteroids, suture-related problems, ocular surface diseases and previous corneal infection.11 Although infection may further complicate recovery, patients with PKIK and rejection are often good candidates for corrective procedures such as Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty.
Sclero-keratoplasty may develop complications based on a number of factors, the most prevalent being graft size. In comparison to small grafts, larger sized corneoscleral grafts were observed to have higher incidences of intraoperative complications and postoperative problems such as issues with sutures, graft failure, graft rejection and the development of secondary glaucoma.13
Medical interventions to improve outcomes of sclero-keratoplasty appear to be promising. Recently, there have been findings to suggest that during postoperative management, immunosuppressants such as mycophenolate mofetil (MMF) and cyclosporine may improve results of sclero-keratoplasty, with results showing significantly improved rejection-free graft survival rates at one year postoperatively.4
With initial use in 1937, Hughes tarso-conjunctival flaps were first developed to correct full-thickness defects in the lower eyelid.8 Most of the success is attributed to the blood supply offered by the upper lid. The use of the flap subsequently evolved into repairing full-thickness defects in the upper lid as well. Today, the flap’s use has been expanded to surgical interventions for inflammatory ocular surface disease. The main goal of flaps in these procedures is to recover the integrity of the corneal surface as well as to prevent gradual corneal ulceration and secondary infection. Patients additionally also experience pain relief, reduced drop burden and enhanced aesthetic appearance, which in many cases has replaced the option of invasive surgery or enucleation.15 This case report describes the integration of a sclero-keratoplasty with a tarso-conjunctival flap in order to treat bacterial keratitis with extensive scleromalacia for an optimized structural outcome.
 |
|
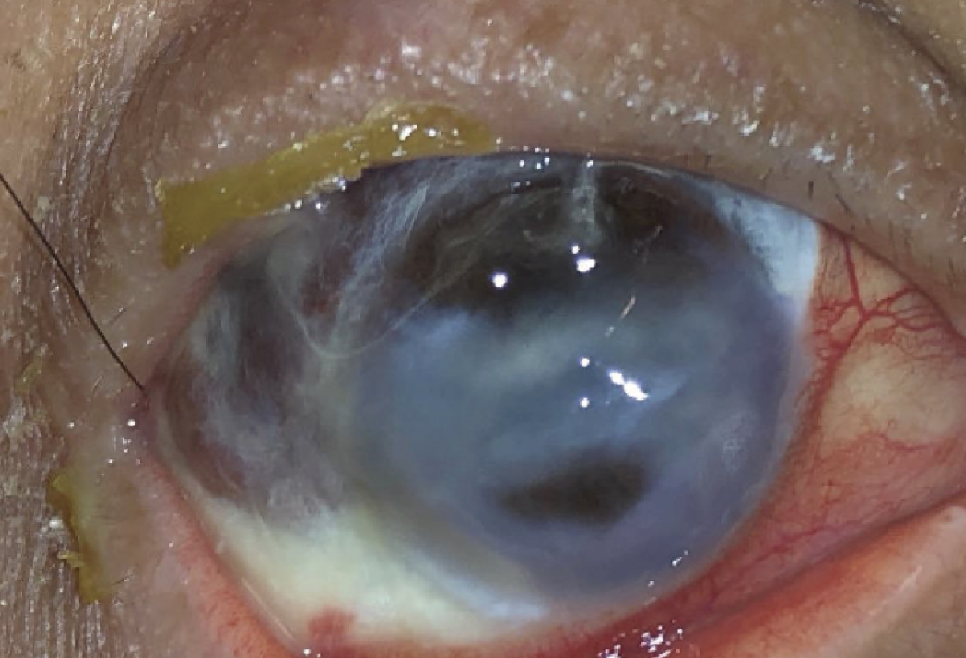
Case 1. Image taken at the preoperative examination. There is superior full thickness corneal melt which is plugged with iris and another smaller full thickness corneal melt just below the visual axis, also plugged with iris. Extensive scleromalacia is noted from 6 o’clock to 2 o’clock. |
Case 1
A 55-year-old female with a medical history of ovarian cancer status post chemotherapy presented with a corneal ulcer of the right eye.
Her presenting illness began at an outside hospital where she was treated with Tobradex, Pred Forte and ketorolac for eye pain and clear discharge. Her symptoms progressed into pain, redness and purulent discharge for which she presented to the emergency room. Her past ocular history is pertinent for extended contact lens use and high myopia.
On clinical examination, a large epithelial defect measuring 10 mm x 6 mm with an area of central coagulative necrosis and extension into the superior sclera was noted. The A/C was deep and formed with a dense hypopyon. B-scan ultrasonography was performed and demonstrated vitritis, which was concerning for endophthalmitis.
For medical management, the patient was hospitalized and initiated on topical vancomycin 25 mg/ml per hour and tobramycin 14 mg/mL per hour. Due to her immunosuppressive state, a broad differential for underlying infectious agents was considered, and the patient was treated with systemic Levaquin for intraocular penetration. She demonstrated improvement with a resolving hypopyon and was stable for discharge.
Due to social circumstances, the patient then followed up in the clinic two weeks post discharge, though she had been compliant on the tapered antibiotic regimen. On exam, the eye appeared grossly quiet with no signs of acute infection. The anterior chamber, however, was flat with a corneal perforation. Due to the extensive scleromalacia from the 6 to 2 o’clock position, a sclero-keratoplasty with an adjunct tarso-conjunctival flap and possible amniotic membrane transplant was recommended.
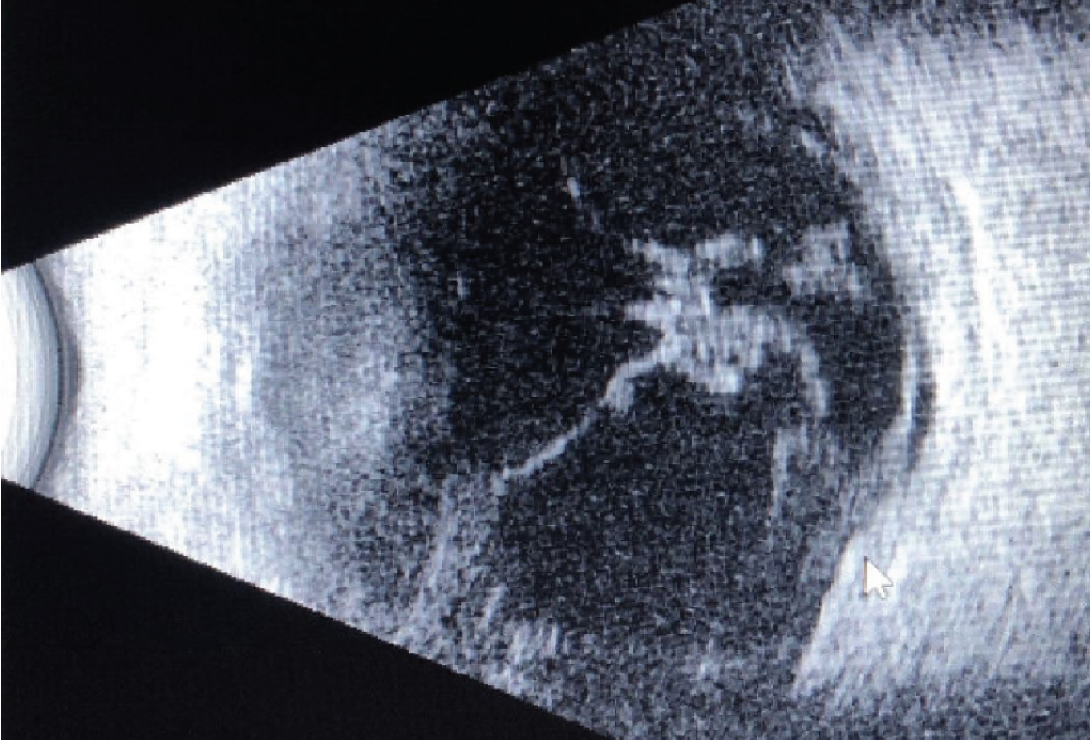 |
| Case 1. B-Scan ultrasonography taken on preoperative examination. Vitreous debris still observed from initial presentation. |
Case 1: Surgical Management
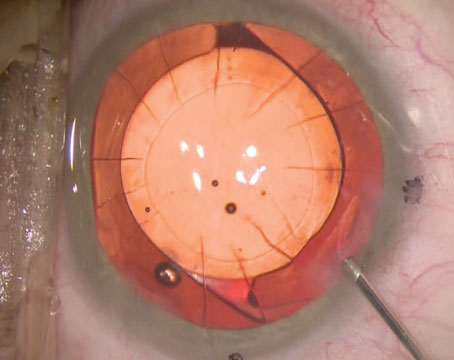
Intraoperatively, a vascular flap from the tarsal conjunctiva was harvested first by everting the upper eyelid and using a #64 blade incision through the horizontal extent of the upper eyelid tarsal plate. This was then dissected posteriorly so as to create a pedicle flap which could be rotated into the correct orientation. Attention was then turned towards securing a full thickness scleral graft from the 6 to 2 o’clock position. A sclero-keratoplasty donor button was sewn into place. Interrupted sutures were used to secure the conjunctival hinge graft, using the pedicle flaps to ensure that the stromal side of the flap approximated the scleral patch graft. The pre-existing conjunctiva was also secured to the new scleral rim. The resulting stromal exposure of the superior bulbar conjunctiva was covered with an amniotic membrane, placed carefully to ensure that the basement membrane was in contact with the surface of the eyelid. An amniotic membrane was also placed over the sclero-keratoplasty, with the basement membrane this time facing towards the globe of the eye. A large contact lens was placed to serve as a barrier to prevent symblepharon formation.
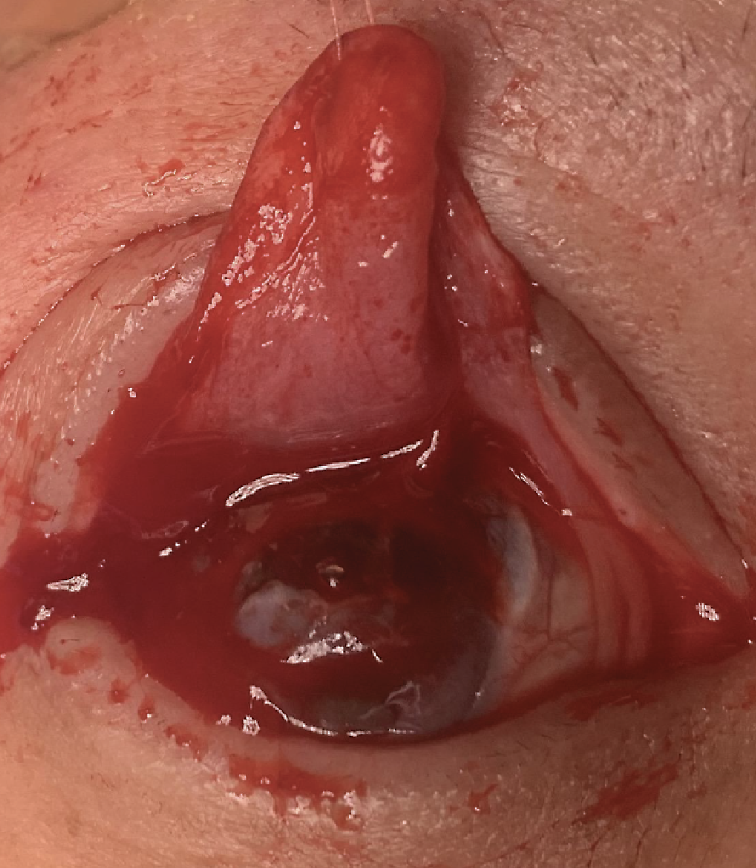 |
| Case 1. Intraoperative photograph of the tarsal conjunctival advancement flap constructed during the procedure. This flap covered the area of scleromalacia from 6 o’clock to 2 o’clock. |
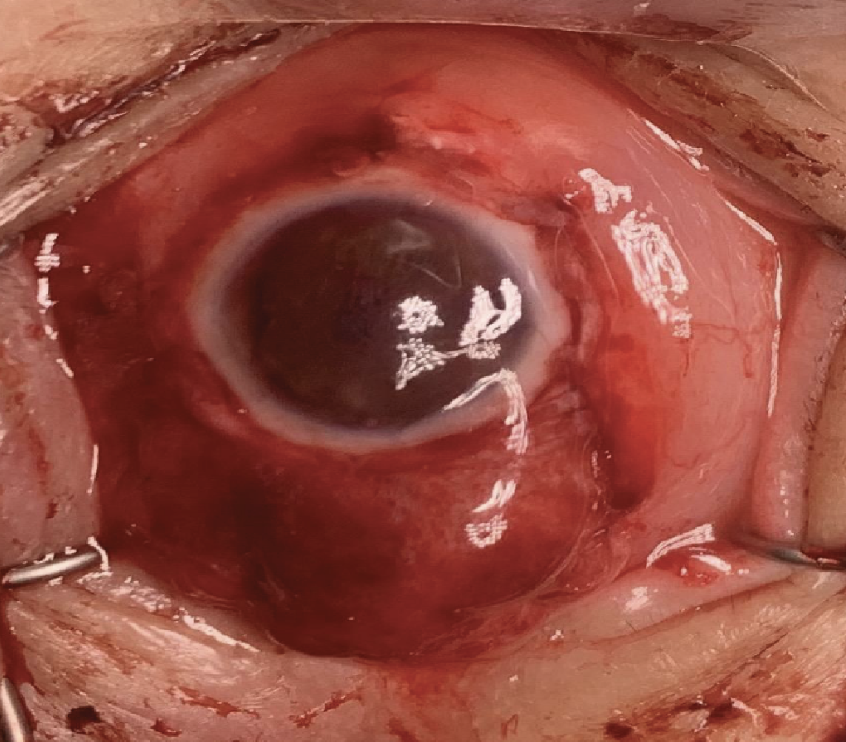 |
| Case 1. Intraoperative photograph of the completed sclero-keratoplasty with placement of a tarso-conjunctival flap. |
Postoperative Follow-up
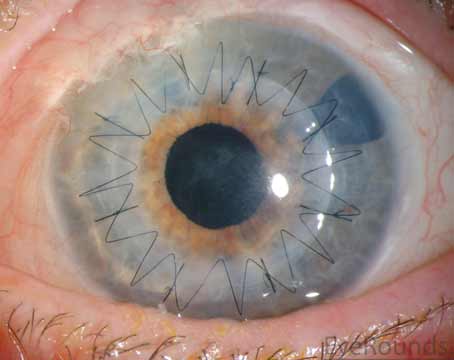
On day one of postoperative follow-up, the visual acuity was 20/200 with a normal intraocular pressure. The anterior chamber was deep and well-formed, and all wounds were Seidel negative. The patient was seen a week later when visual acuity was reduced to hand motion (HM), notably due to a hyphema and a pupillary membrane. At the one-month follow-up, vision improved to count fingers at the distance of 1 foot. The hyphema and anterior chamber reaction appeared to be resolved, however, the pupillary membrane was obstructing visual potential. Overall, the graft was intact and the cornea appeared clear.
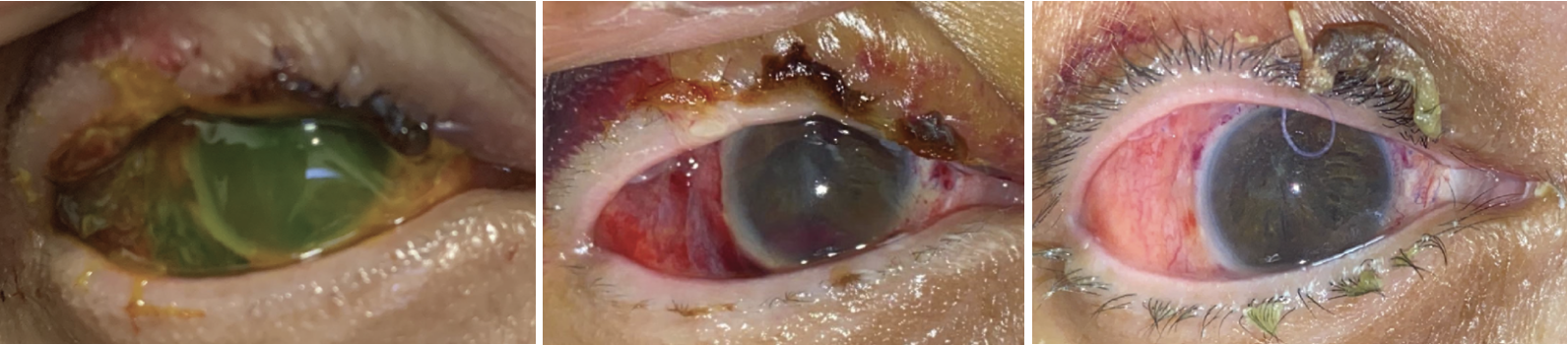 |
| Case 1. (Left) External photograph of the right eye on primary gaze one day postoperatively. Anterior chamber is deep and the amniotic membrane is intact covering the surface. (Middle) External photograph of the right eye in primary position taken at one week postoperatively. The amniotic membrane has melted. Anterior chamber is deep with resolving anterior chamber hyphema. (Right) External photograph of the right eye taken one month postoperatively. The corneal surface is intact and the stroma is clear. Pupillary membrane was noted in visual axis. There’s no sign of infection persistence. |
Case 2
54-year-old female with history of Stevens Johnson syndrome (SJS) and complicated ocular history including placement of Xen gel stent with use of mitomycin C developed scleral melt following endophthalmitis. She developed pain, photophobia and injection of the left eye and on exam was found to have layered hypopyon with vitritis consistent with endophthalmitis. This was thought to be related to the Xen gel stent which had been placed nine months prior as she had a previous episode of endophthalmitis in the same eye.
She was started on medical treatment including Vigamox and tobramycin every two hours, moxifloxacin 400 mg daily (although the patient refused to take this due to concern for SJS), Tobradex ointment as needed, and Durezol every two hours. She was taken that afternoon for removal of the Xen stent with vitreous tap and injection of 0.1 cc of vancomycin and 0.1 cc of ceftazidime. She also received subconjunctival injections of dexamethasone and cefazolin. The vitreous cultures didn’t grow any organisms.
She was followed closely and over the following month the steroids and antibiotics were tapered appropriately. Unfortunately, at a follow-up visit one month after removal of the Xen gel stent, scleral melt was noted. A Kontour lens was placed and the Durezol and Vigamox were increased to four times daily. She was also started on valacyclovir 1,000 mg twice daily.
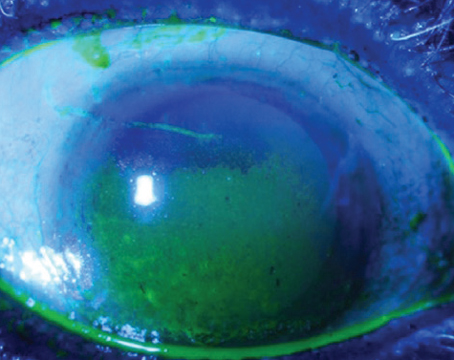
She continued to be followed closely, and was taken for amniotic membrane grafting to the area of scleral melt. However, within three weeks the amniotic membrane failed to produce sufficient healing and an avascular area of scleromalacia was progressing. The decision was made to perform a tarso-conjunctival flap to promote healing.
Case 2: Surgical Management
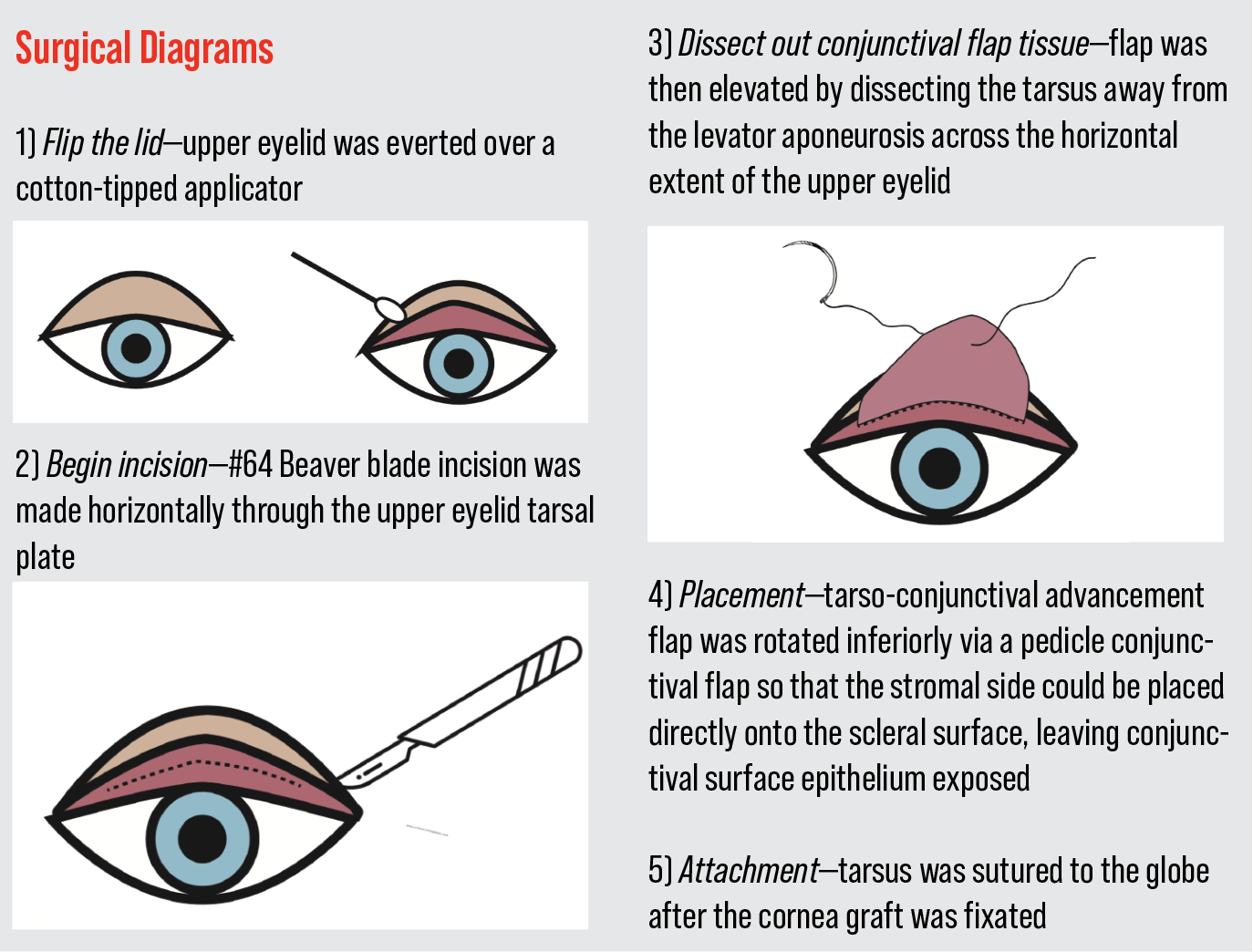
The upper eyelid was everted over a cotton tip applicator (See Surgical Diagrams on p. 70). A #15 Beaver blade was used to incise horizontally through the upper eyelid tarsal plate. A tarso-conjunctival advancement flap was created by dissecting between the tarsus and the levator aponeurosis across the horizontal extent of the upper eyelid. This was then rotated inferiorly onto the necrotic sclera and sutured into place. Amniotic membrane was sutured into place to cover the flap and was extended posteriorly into the fornix. This was further secured with Tisseel fibrin glue. A single tarsorrhaphy suture was placed to immobilize the lids and promote healing.
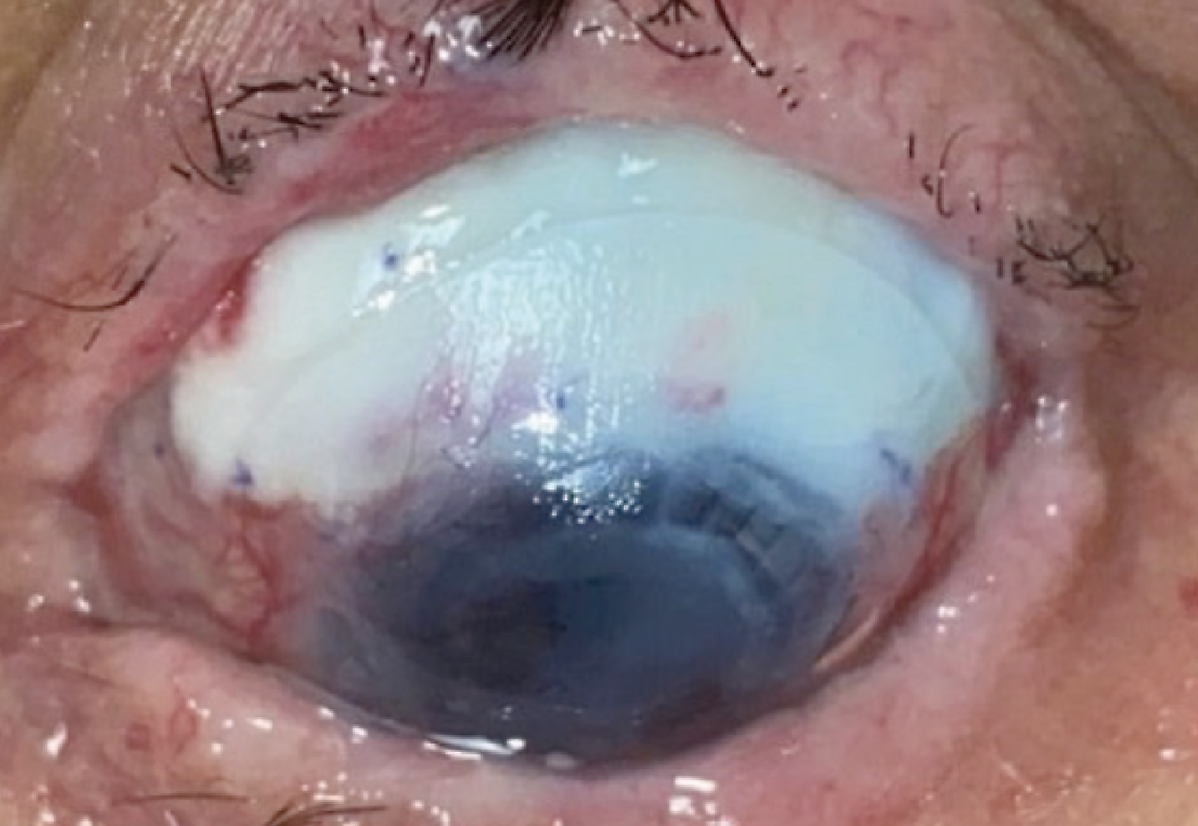 |
| Case 2. Preoperative superior scleromalacia with avascular sclera and Kontour lens in place. |
Postoperative Follow-up
On a postoperative-day-one telemedicine visit, the patient was doing well. However, a week later, the vision had decreased from hand motion to light perception and the flap had dehisced. Re-suturing was performed in the operating room the next day, and the flap remained secure.
Discussion
The use of a tarso-conjunctival flap has been quite impactful in ocular surface surgeries, allowing patients to experience improved healing. Although use is primarily indicated in non-infectious ocular surface diseases, application in bacterial keratitis with extensive scleromalacia can be beneficial. Use of a flap may reduce the size of the graft needed, thereby lowering failure rates of scelero-keratoplasty grafts.13 In addition to reducing size, tarso-conjunctival flaps serve as a source of vascularized tissue for the cornea. Increased blood flow and lymphatics account for a remarkable increase in growth factors and cellular components that prime the corneal surface for repair. The result is an increased resistance to anti-collagenolytic substances that prevent subsequent stromal ulceration. With regard to inflammation, tarso-conjunctival flaps have further shown to prevent pro-inflammatory mediators from reaching the affected area, leading to decreased instances of stromal lysis.1,10,12 Use of a tarso-conjunctival flap is of the utmost importance in instances where other conventional methods may not be possible. Although amniotic membranes have been shown to be largely advantageous in sclero-keratoplasty cases by substantially facilitating epithelial closure, they may not always be readily available.9 In these circumstances, the use of a conjunctival flap can be used as a low-risk measure to facilitate healing and provide coverage to all scleral areas.
Dr. Alapati is a uveitis fellow at Northwestern University. Dr. Miller O’Dell practices at the Oklahoma City Veterans Hospital. Mr. Morcos is medical student at the University of Missouri-Kansas City. Dr. Goins is a professor of ophthalmology at the University of Kansas. Dr. Sokol is the interim chair of ophthalmology at the University of Kansas.
1. Abdulhalim BE, Wagih MM, Gad AA, Boghdadi G and Nagy RR. Amniotic membrane graft to conjunctival flap in treatment of non-viral resistant infectious keratitis: A randomised clinical study. Br J Ophthalmol 2015;99:59–63.
2. Al-Mujaini A, Al-Kharusi N, Thakral A, Wali U. K. Bacterial keratitis: Perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos University Medical Journal 2009;9:2:184–195.
3. Amescua G, Miller D, Alfonso EC. What is causing the corneal ulcer? Management strategies for unresponsive corneal ulceration. Eye (London, England) 2012;26:2:228–236.
4. Bali S, Filek R, Si F, Hodge W. Systemic Immunosuppression in high-risk penetrating keratoplasty: A systematic review. Journal of Clinical Medicine Research 2016;8:4:269–276.
5. Bennett JE, Dolin R, Blaser MJ. Microbial keratitis. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia: Elsevier, 2020.
6. Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, Beach MJ, Centers for Disease Control and Prevention (CDC). Estimated burden of keratitis—United States, 2010. Morbidity and Mortality Weekly Report 2014;63:45:1027-1030.
7. Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP. Amniotic membrane transplantation in the human eye. Deutsches Arzteblatt International 2011;108:14:243–248.
8. Rohrich RJ, Zbar RI. The evolution of the Hughes tarsoconjunctival flap for the lower eyelid reconstruction. Plast Reconstr Surg 1999;104:2:518-22;523 (quiz), 524-6 (discussion).
9. Seitz B. Amniotic membrane transplantation. An indispensable therapy option for persistent corneal epithelial defects. Ophthalmologe 2007;104:10.
10. Sharma A, Mohan K, Sharma R, Nirankari VS. Repositioning of pedicle conjunctival flap performed for refractory corneal ulcer. Middle East Afr J Ophthalmol 2014;21:89–91.
11. Song A, Deshmukh R, Lin H, Ang M, Mehta JS, Chodosh J, Said DG, Dua HS, Ting DSJ. Post-keratoplasty infectious keratitis: Epidemiology, risk factors, management, and outcomes. Front Med 2021;8:707242.
12. Stamate AC, Tătaru CP, Zemba M. Update on surgical management of corneal ulceration and perforation. Rom J Ophthalmol 2019;63:166–173.
13. Thatte S, Dube AB, Dubey T, Krishnan M. Outcome of sclerokeratoplasty in devastating sclerocorneal infections. J Curr Ophthalmol 2020;32:1:38-45.
14. Wong RL, Gangwani RA, Yu LW, Lai JS. New treatments for bacterial keratitis. Journal of Ophthalmology 2012, 831502. https://doi.org/10.1155/2012/831502
15 Zemba M, Stamate AC, Tataru CP, Branisteanu DC, Balta F. Conjunctival flap surgery in the management of ocular surface disease (review). Experimental and Therapeutic Medicine 2020;20:4:3412–3416.