Corneal transplantation has moved from the back stage to center stage in the arena of modern-day ophthalmic surgery. We focus on three major areas when we talk about conventional full-thickness corneal transplantation: the corneal wound; graft rejection and postoperative quality of vision. When we shift from full-thickness to partial-thickness corneal transplantation, we introduce a fourth factor—the donor-recipient corneal interface.
 The Biomechanics of PK
The Biomechanics of PK
Penetrating keratoplasty involves a full-thickness wound. Biomechanically speaking, this type of wound weakens the patient’s cornea. In fact, the wound itself becomes the weakest part of the cornea, which can rupture when the eye is subjected to direct trauma. This scenario becomes even more significant in a monocular patient. Additionally, with PK we introduce a foreign material—sutures—to hold the donor cornea in place during healing. Sutures can contribute to subsequent infection and can break, causing emergent eye pain requiring immediate attention. Such a full-thickness wound also induces iatrogenic corneal astigmatism.
The Potential of Lasers
The use of laser technology in full-thickness corneal transplantation has brought some new advantages to the procedure. For example, lasers provide for greater speed, better reproducibility and more variations in the patterns of wound construction. However, laser use has not eliminated the need for sutures, nor has it eliminated astigmatism.
However, lasers are thought to provide faster wound healing and earlier suture removal compared to manual wound construction. So the question then becomes: Is laser surgery worth the added cost? Additionally, in most cases Intralase enabled keratoplasty (IEK) involves two sites: one for laser wound construction and the second for the manual portion of the keratoplasty.
In full-thickness corneal scars involving the central cornea, the choice of surgical procedure is often limited to a full-thickness PK. You can discuss both the manual and laser techniques with the patient and decide if manual PK or IEK is warranted.
Partial-Thickness Options
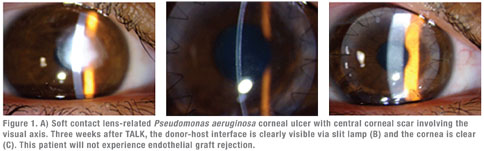
You can consider partial-thickness procedures when the corneal disease process does not involve the full thickness of the cornea. The type of partial-thickness keratoplasty procedure will depend on the location of corneal opacity. When the opacity is in the front part of the cornea, we can perform anterior lamellar keratoplasty. The John-Malbran classification divides ALK into four types of procedures: superficial ALK (SALK), mid-ALK (MALK), deep-ALK (DALK) and total-ALK (TALK) procedures. When the opacity is in the back part of the cornea, we can perform posterior lamellar keratoplasty (PLK) or procedures such as Descemet’s stripping automated endothelial keratoplasty (DSAEK) or Descemet’s membrane endothelial keratoplasty (DMEK).
With regards to ALK procedures, we can perform SALK without corneal sutures while the remaining ALK operations usually require corneal sutures to hold the donor cornea in place during wound healing. These partial-thickness procedures introduce the donor-host interface, which involves the central visual axis and hence will affect the patient’s vision. Add in the time factor for the interface to gradually clear and improve the patient’s vision. Remember that the deeper the donor-host interface, the better the quality of vision.
Endothelial Procedures
When the disease process involves the innermost corneal layer, mainly the endothelium, then DSAEK or DMEK become options—depending on the surgeon’s preference and comfort level. In my opinion, DMEK is far superior to DSAEK on almost all scores, including visual acuity (potential for 20/20 or better); faster visual recovery; better quality of vision; and restoration of the normal corneal anatomy.
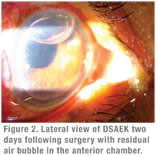 DSAEK adds tissue to the cornea, so the patient’s cornea becomes thicker than normal. DMEK, on the other hand, substitutes tissue in the cornea, resulting in normal corneal thickness. Additional concerns with DSAEK include the donor tissue margin of 360 degrees. Unlike any other organ transplantation procedure in the human body, DSAEK corneal transplants have corneal stromal graft edges exposed to the aqueous humor. There is no donor-to-recipient tissue margin approximation and hence no possibility of tissue cell-to-cell contact inhibition.
DSAEK adds tissue to the cornea, so the patient’s cornea becomes thicker than normal. DMEK, on the other hand, substitutes tissue in the cornea, resulting in normal corneal thickness. Additional concerns with DSAEK include the donor tissue margin of 360 degrees. Unlike any other organ transplantation procedure in the human body, DSAEK corneal transplants have corneal stromal graft edges exposed to the aqueous humor. There is no donor-to-recipient tissue margin approximation and hence no possibility of tissue cell-to-cell contact inhibition.
DMEK brings a lot of advantages to the modern-day keratoplasty procedures. However, this procedure is technically more challenging for the corneal surgeon than DSAEK. With DMEK, the surgeon must be mindful to not accidentally tear the donor Descemet’s membrane, since it will result in using a second donor cornea and add to the surgical cost. DMEK also involves properly unraveling the rolled-up donor Descemet’s membrane within the anterior chamber and properly orienting the donor Descemet’s membrane to the recipient corneal stroma. Finally, the surgeon must hold the donor Descemet’s membrane in place with a large air bubble.
Modern-day corneal transplant surgeons have a larger menu of procedures to choose from to provide the optimal postop vision for their patients. Keeping in mind the role of corneal biomechanics, the better procedure may ultimately be one that continues to provide the best corneal biomechanics and the best quality of vision that patients can enjoy for the rest of their lives.
Dr. John is an ophthalmic surgeon and cornea specialist in private practice in Oak Brook, Tinley Park and Oak Lawn, Ill. He is a clinical associate professor at Loyola University at Chicago, and visiting professor, Department of Defense, Military Medical Academy, Belgrade. Contact him at tjcornea@gmail.com. He has no financial interest in any aspect of this article.
1. Barraquer JI. Keratoplasty. CVI Concilium Ophthalmologicum, Britannia. 1950;2:999.
2. Tillet CW. Posterior lamellar keratoplasty. Am J Ophthalmol 1956;41:530-533.
3. Melles GR, Eggink FA, Lander F, Pels E, et al. A surgical technique for posterior lamellar keratoplasty. Cornea 1998;17:618-626.
4. Melles GRJ, Lander F, Beekhuis WH, Remeijer L, et al. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratoplasty. Am J Ophthalmol 1999;127:340-341.
5. John T. Selective tissue corneal transplantation: A great step forward in global visual restoration. Expert Rev Ophthalmol 2006;1:5-7.
6. John T. Surgical Techniques in Anterior and Posterior Lamellar Keratoplasty. New Delhi, India: Jaypee Bros. Medical Publishers; 2006.
7. John T. Step by Step Anterior and Posterior Lamellar Keratoplasty. New Delhi, India: Jaypee Bros. Medical Publishers; 2006.
8. John, T (Ed). Lamellar Corneal Surgery. New York, NY: McGraw-Hill Companies; 2008.
9. John, T (Ed). Endothelial Transplant, DSAEK, DMEK, & DLEK. New Delhi, India: Jaypee-Highlights Medical Publishers Inc.; 2010.
10. John, T (Ed): The Chicago Eye and Emergency Manual. New Delhi, India: Jaypee-Highlights Medical Publishers Inc.; 2011.
11. Price MO, Gorovoy M, Benetz BA, et al. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology 2010;117:438-444.
12. John T, Taylor DA, Shimmyo M, Siskowski BE. Corneal hysteresis following descemetorhexis with endokeratoplasty: Early results. Ann Ophthalmol (Skokie) 2007;39:9-14.
13. Terry M. The evolution of lamellar grafting techniques over twenty-five years. Cornea 2000;19:611-616.
14. Busin M, Arffa RC, Sebastiani A. Endokeratoplasty as an alternative to penetrating keratoplasty for the surgical treatment of diseased endothelium initial results. Ophthalmology 2000;107:2077-2082.
15. Brunette I. Evolution in surgical techniques and indications for corneal transplantation: past, present, and future. Can J Ophthalmol 2011;46:297-299.