Types of Stem Cells
Mammalian stem cells may be A) embryonic stem cells, B) adult stem cells or C) cord blood stem cells. The inner cell mass of blastocysts contains the embryonic stem cells, while the adult stem cells are housed in various tissues of the body, where they (adult stem cells and progenitor cells) replace adult tissues as a reparative process of the body. Unlike the adult scene, in the developing embryo, these stem cells are pluripotent and can differentiate into ectoderm, mesoderm and endoderm, thus covering all the bodily specialized cells.
• Stem cell origin. Adult, autologous stem cells can originate from the bone marrow, blood and fat or adipose tissue. For bone-marrow-derived stem cells, drilling is carried out at the iliac crest or the femur, while blood-derived-stem cells can be obtained by apheresis harvest of stem cells from donor blood. On the other hand, stem cells from adipose tissue are obtained by liposuction. Yet another source of stem cells can be the umbilical cord blood immediately after birth.
• Stem cell features. To qualify as a stem cell, cells have to self-renew with potency to differentiate into specialized cells. Self-renewal refers to the ability of the cells to undergo multiple cell division cycles while at the same time maintaining their undifferentiated cell state. From a potency standpoint, the stem cells have to be pluripotent or totipotent to be able to form any mature cell type. The various types of stem cell potency are described below:
Pluripotent: These stem cells are derived from totipotent stem cells and can differentiate into any of the three germ layers.1
Totipotent or omnipotent: These are stem cells with the maximum potential, namely they can differentiate into both embryonic and extra-embryonic cell types. Biologically, they can build a complete organism that is viable. These totipotent stem cells are produced as a result of the fusion between an egg and sperm cell and also from the initial few divisions of the fertilized egg.2
Multi-potent: These stem cells can differentiate into a number of different cells that are closely related to a family of cells and they are also in the amniotic fluid.3
Oligo-potent: These stem cells have limited potential and can differentiate into a few cells, for example, myeloid or lymphoid stem cells.3
Uni-potent: These stem cells can produce only their own type of cells (one cell type).3 The property of self-renewal differentiates these cells from non-stem cells.
Induced pluripotent: These are different from all the stem cells described above. These are adult cells such as epithelial cells that are then reprogrammed to acquire pluripotent potential. These often involve genetic reprogramming using protein transcription factors.
Ocular Stem Cells
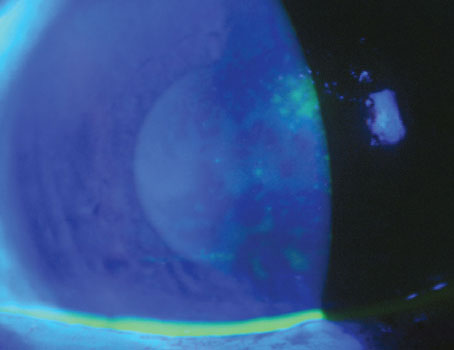
Treating ocular diseases utilizing stem cells is of significant interest to both clinicians and researchers. Currently, there is no treatment to fully cure ocular surface stem cell deficiency states and ocular neurodegenerative diseases. Ocular location of stem cells is primarily in the front regions of the globe, namely, at the limbus, conjunctiva and retinal-ciliary margin. Limbal stem cells are sandwiched between conjunctival epithelium on the outside and corneal epithelium on the inside. Among all adult somatic stem cells, those of the corneal epithelium have a unique location in a specific limbal structure called the Palisades of Vogt.4 The understanding that corneal epithelial stem cells are located in the limbal area sheds light on multiple observations including the mature-looking basal cells, the preponderance of tumor formation in the limbal zone, and the centripetal cellular migration.5
Limbal stem cells play an important role in corneal homeostasis. This garland-like pattern of the limbal, corneal epithelial stem cells on the ocular surface, encircling the limbus, also plays a role in policing the area, thus, preventing the conjunctival epithelium from encroaching onto the clear cornea, which can result in corneal neovascularization, corneal clouding and blurred vision or even total vision loss. Human limbal stem cells detection is possible both in vivo and in vitro by their expression of p63 transcription factor. Protein p63 retains the proliferative potential of limbal stem cells. Additionally, extraocular stem cells, namely, adult stem cells, embryonic stem cells and induced pluripotent stem cells seem to be promising in restoring vision loss as well.
|
Moving to the back of the eye, in the retinal arena, there is heightened interest in the potential for stem cell treatment in retinal degenerative diseases such as age-related macular degeneration, Stargardt’s disease and retinitis pigmentosa, which lack curative treatment at the present time. Many of the outer retinal diseases seem to be linked to the degeneration of the epithelial monolayer, namely, the retinal pigment epithelium, which is essential for supporting tissue involved in retinol cycling, nutrient transport, growth factor production and phagocytosis of the photoreceptor outer segments.6
Management
It is conceivable that the treatment of human diseases can be revolutionized by stem cell therapy. Although there are various treatment strategies, one widely known approach is cell replacement therapy, in which stem cells that are differentiated into the desired cell type are then delivered to the damaged tissue in order to integrate and restore function.6 An alternative modality is via a paracrine effect, in which the transplanted stem cells secrete trophic factors that help the resident tissue to self-restore and proliferate.6,7 Further, there is some evidence that stem cells may fuse with existing cells in order to restore function.6,8
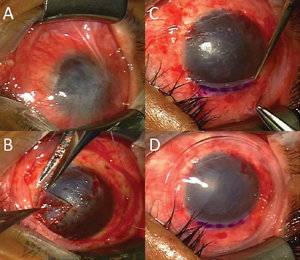
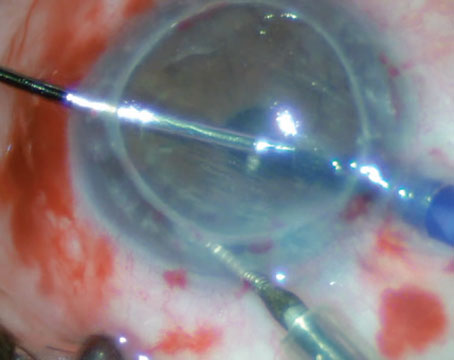
The risk with the use of stem cells is decreased when we use autologous harvesting, obtaining the stem cells from one’s own body. The various choices of limbal stem cell restoration include 1) keratolimbal allograft from a donor cornea; 2) conjunctival allograft from a living relative; 3) patient’s conjunctival limbal autograft; and 4) autologous culture of limbal cells used as a route of transplant tissue. However, the culture techniques are currently not FDA-approved; hence the other options may be considered based on unilateral or bilateral ocular involvement, and the severity of the limbal stem cell deficiency. Following limbal stem cell stabilization, any corneal opacity that is interfering with vision may be addressed by partial thickness, anterior, lamellar keratoplasty, penetrating keratoplasty or a keratoprosthesis (artificial cornea) such as the Boston keratoprosthesis.
Stem cell-based treatment for retinal disease states bifurcates into regenerative and trophic approaches, both of which comprise the implantation of cells into the subretinal space. In the regenerative strategy, a human embryonic stem cell (hESC) line that has differentiated into retinal pigment epithelial cells is used to replace the disease-related, RPE cell loss. In the trophic approach, undifferentiated human umbilical, tissue-derived cells (hUTC) are used to support degenerating cells via the release of different mediators including neurotrophic factors, interleukins, etc.
Complete success in the arena of human stem cell replacement would result in a paramount effect on anatomic and functional tissue restoration, with relief of stem cell deficiency related ocular symptoms and regaining vision. REVIEW
Dr. John is an ophthalmic surgeon and cornea specialist in private practice in Oak Brook, Tinley Park and Oak Lawn, Ill. He is a clinical associate professor at Loyola University at Chicago, and visiting professor, Department of Defense, Military Medical Academy, Belgrade. Contact him at tjcornea@gmail.com.
1. Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng 2005;100:12-27.
2. Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol 2009;114:185-99.
3. Schöler H. The Potential of Stem Cells: An Inventory. In: Knoepffler N, Schipanski D, and Sorgner S, eds. Humanbiotechnology as Social Challenge. Surry, UK: Ashgate Publishing, 2007:28.
4. Li W, Hayashida Y, Chen Y, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res 2007;17:26-36.
5. Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: Looking at some old problems from a new angle. Exp Eye Res 2004;78:433-46.
6. Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: Past, present and future. Development 2013;140:2576-2585.
7. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 2012;3:359.
8. Ying Q, Nichols J, Evans E, Smith A. Changing potency by spontaneous fusion. Nature 2002;416:545-548.