Mark Abelson, MD, CM, FRCSC and Mia Benenate,
Mycotic keratitis is an infection of the cornea caused by yeast or filamentous fungi infiltrating the epithelium. It continues to pose diagnostic problems for physicians because the clinical presentation of a fungal infection is not specific to the fungi genus.
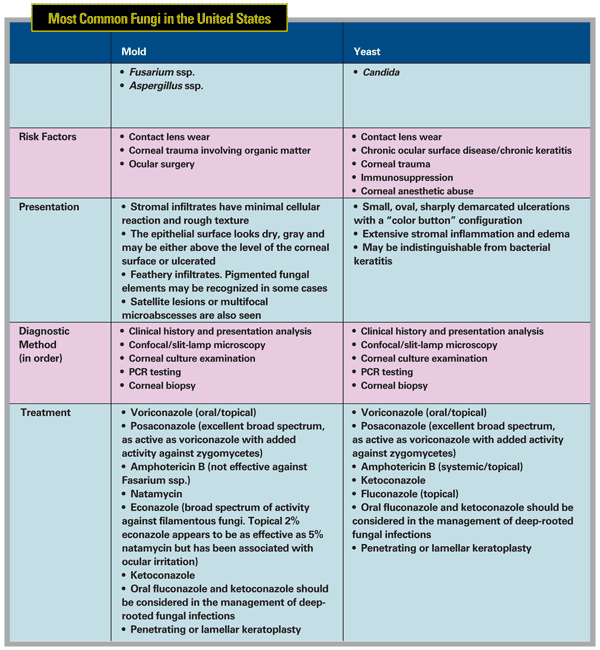
There are, however, significant clinical and epidemiological differences between mold and yeast pathogens themselves, primarily manifesting in predisposing factors and clinical features of the resulting infections.
Although fungal pathogens are numerous and varied, the most common etiologic agent of filamentous fungi in the
The most common fungal agent for yeast is Candida. Less common pathogens found in the
Because of the comparative rarity of mycotic keratitis, and the fact that its symptoms mirror those of more common bacterial infections, frequently the detection of the infection is weeks late and, therefore, the patient receives a less effective treatment. The importance of early identification must be stressed; treatment has proven to be most effective if aggressively administered in the early stages of infection. If the keratitis is allowed to progress, the risk of needing an invasive keratoplasty rises as the integrity of the ocular structure and visual function is threatened in the infected eye.
Prevention
Preventing mycotic keratitis is easier than might be expected. When discussing prevention with patients, stress good ocular hygiene, proper safety precautions and general awareness when outdoors. Principally, prevention of this infection comes down to a general awareness of the disease itself and its inherent risk factors. The three main risk factors in the
The potential for developing mycotic keratitis is heightened when the lens wearer does not follow an optimal routine, and lens-care hygiene is one of the best tools we have for preventing cases of mycotic keratitis. Habits such as infrequent lens cleaning should be discouraged, and positive steps such as appropriate cleaning and maintenance regimens that minimize risk must be emphasized. Stress the importance of washing hands before and after removing or placing contacts into the eye. Make sure that the patient is aware of the risks involved with soft contact lenses and extended-wear lenses. If patients prefer extended-wear lenses, make sure they know that they should be removing and cleaning the lenses at least once a day to minimize the risk of infection. Patients should also clean their contact lens cases regularly after each use and use new solution from the bottle.
Contact lens solution should never be allowed to sit for hours or days and then be used again for another cleaning. Lenses should also be removed prior to showering.
Another important preventive measure to stress is caution and general awareness while outdoors, especially for agricultural workers in the southern
Rapid Detection and Diagnosis
The clinical diagnosis of mycotic keratitis is based on risk-factor analysis, characteristic features and, most importantly, on cultures taken from corneal scrapings. When diagnosing mycotic keratitis, it is extremely important to consider information gathered during all investigative queries. The patient will often complain of one or more of the following symptoms: ocular irritation and pain; dry eye; increased light sensitivity; watery discharge; possibly a foreign body sensation; and often, sudden blurry vision or a decrease in vision in the eye.5 Be especially suspicious of mycotic keratitis if the patient has sustained corneal trauma, wears contact lenses, is immunocompromised, is in poor general health or has an agricultural job.
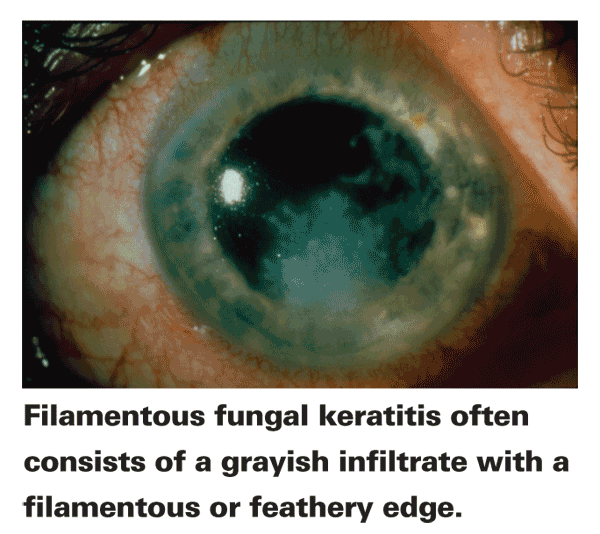
If you're suspicious of a fungal infection after the initial consultation, the next step is an examination of the corneas using slit-lamp or confocal microscopy (if available at a nearby academic center); both methods allow an in-depth, detailed look at the ocular structures. This can reveal physical characteristics of mycotic keratitis and help the physician determine corneal damage. The presentation of mycotic keratitis can include a grayish, dry-looking epithelial layer, rough textured stromal infiltrates that may rest above the level of the corneal surface and are possibly ulcerated, as well as infiltrates with feathery borders, depending on the pathogen. Also look for the presence of satellite lesions, hypopyon, epithelial plaque, multifocal microabscesses, extensive inflammation and edema.
Visual confirmation of any of the above symptoms should prompt an immediate culture taken from corneal scrapings, as there is not a more accurate method than the collection of specimens for cultural and histological identification. These symptoms, combined with a suspicious clinical history such as ocular trauma involving vegetative matter or soil, et cetera, should prompt an immediate therapeutic response with a broad-spectrum antifungal.
Specimens can be obtained by scraping directly on the corneal surface using a surgical blade, sharpened platinum spatula or calcium alginate swab and then inoculated on agar plates. Once the media has been set, specimens should be maintained at 25 degrees Celsius to encourage fungal growth. The cultures should be held for a minimum of four weeks; fungal cultures can test positive for mycotic keratitis in a matter of days or take as long as six weeks, though initial growth in 83 percent of fungal cultures occurs within the first 72 hours of incubation and 97 percent within the first week.
Perhaps the quickest way to a diagnostic answer for mycotic keratitis is using the polymerase chain reaction test,1 which identifies fungi, bacteria and viruses through a thermal cycling process. This technique is helpful in replicating the relatively small corneal specimens into a larger sample for an analysis of fungal pathogens. There have been promising results in detecting mycotic keratitis using FTA filter paper for specimen collection along with PCR, and clinical specificity is between 91.7 and 99 percent.6 Results from PCR are evident between four and 24 hours; however, the downside of this rapid and inexpensive testing method is that it has been associated with a high false-positive rate.6
If all tests are negative after 48 to 72 hours and there is still a strong suspicion of fungal infection, it may be necessary to perform a corneal biopsy, in which case the specimen should be submitted to the laboratory and a sample submitted for histopathologic examination. The glass slides of the histology exam may reveal the hyphae specific to filamentous fungi or characteristics specific to yeast fungi under the microscope. The pathologist should be alerted regarding the suspected diagnosis and especially that the specimen is a small piece of cornea.7
While searching for a definitive diagnosis of mycotic keratitis, it's extremely important to ensure that it's not another infection with similar characteristics such as bacterial keratitis, Acanthamoeba keratitis, which is a parasitic infection; or endophthalmitis keratitis. You can rule out the last entity with a B-scan ultrasound.8 Continue with the broad-spectrum antifungal until the specific fungal pathogen is identified.
Treatment
Once there is a definitive diagnosis of mycotic keratitis, the best opportunity for recovery is an aggressive and continuous antifungal treatment that's specific to the pathogen identified. Fungal infections can often be somewhat resistant to medical therapies, both systemic and topical, and penetrate deeper into the cornea than other types of infections. Fungal infections can also be challenging to treat due to a combination of the characteristics of the pathogen, the limited availability of effective therapy, and poor tissue penetration of those therapies.5 Systemic and topical steroids should generally be avoided as an adjunctive therapy to antifungal treatments because they present the risk of inflammation and exacerbation of the fungal infection.5
There are four different groups of antifungal therapies used to fight all localized fungal infections: polyenes; azoles; allylamine and echinocandins.9 The two most commonly prescribed antifungal treatments are amphotericin B, the oldest antifungal treatment primarily effective against Candida pathogens, and natamycin, which is the drug of choice against filamentous fungi. More recently, however, studies have shown that triazoles—and more specifically voriconazole, a broad-spectrum antifungal agent effective against yeasts and molds—may be more effective (in vitro) than natamycin and amphotericin B against fungi; they are sometimes used in conjunction with both.4,8,9-12
Unfortunately, most of the available antifungal treatments are fungistatic and require a prolonged course of action.5 If caught in the early stages, there's a good chance of managing mycotic keratitis without surgery. The goal is to choose the best course of therapy based on the clinical culture results to promote epithelial and stromal healing as well as structural integrity. Along with topical and systemic therapies, frequent localized debridement is recommended to remove superficial corneal necrotic tissue from the infection site.
Untreated mycotic keratitis can lead to permanent vision loss. Even in cases treated appropriately, 15 to 27 percent of patients will require a therapeutic penetrating keratoplasty or lamellar keratoplasty before there's a recession of the fungal pathogen.
Because of the often late and inaccurate diagnosis of mycotic keratitis, some patients who undergo a keratoplasty will be visually impaired.
No new medications for mycotic keratitis have been approved in the
Obviously, there is a pressing need for additional research and development in this area of medical science. Until such research bears fruit, we must ensure that we're able to recognize the signs of even the rarest of pathogens, so that we're prepared to treat cases when they arise.
Dr. Abelson, an associate clinical professor ophthalmology at
1. Zunaina E, Wan HWH, Chan YY, et al. Specific detection of fungal pathogens by I8S rRNA gene PCR in microbial keratitis. BMC Ophthalmology 2008. Accessed 19 May 2010. Available at: http://www.biomedcentral.com/content/pdf/1471-2415--8-7.pdf
2. Qiu WY, Yao YF, Zhu YF, Zhang YM, Zhou P, Jin YQ, Zhang B. Fungal spectrum identified by a new slide culture and in vitro drug susceptibility using Etest in fungal keratitis. Current Eye Research 2005;30:1113-1120.
3. Mack RJ, Shott S, Schatz S, Farley SJ. Association between moxifloxacin ophthalmic solution and fungal infection in patients with corneal ulcers and microbial keratitis. Journal of Ocular Pharmacology and Therapeutics 2009;25:279-283.
4. Afshari NA, Chiatogu O, Heitman J. Calcineurin promotes infection of the cornea by candida albicans and can be targeted to enhance fluconazole therapy. ASM 2006;50:3963-3965.
5. Azar A, Miller B. Principals and Practice of Ophthalmology. 3rd ed. vol. 1.
6. Menassa N, PP Bosshard, Kaufmann C, et al. Rapid detection of fungal keratitis using DNA-stabilizing FTA filter paper. IOVS Papers in Press, 2010;51:1905-1910.
7. Daljit S, Arun V. Keratitis, fungal. eMedicine Ophthalmology 2008. Accessed 19 May 2010. Available at: http://emedicine.medscape.com/article/1194167-print
8. Weiyun S, Wang T, Xie L, Li S, et al. Risk factors, clinical features and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology 2010;117:890-896.
9. Al-Badriyeh D, Neoh CF, Stewart K, Kong DCM. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clinical Ophthalmology 2010;4:391-405.
10. Bunya VY, Hammersmith KM, Rapuano CJ, et al. Topical and oral voriconazole in the treatment of fungal keratitis. Amer J Ophthalmol 2007;143:151-153.
11. Thiel MA, Zinkernagel AS, Burhenne J, et al. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrobial Agents and Chemotherapy. 2007;51:239-244.
12. Wang LLZ, Li R, Luo S, Sun X. In vitro evaluation of combination antifungal activity against fusarium species isolated from ocular tissues of keratomycosis patients. Amer J Ophthalmol 2008;146:724-728.