Miotic eye drops are traditionally used for the treatment of glaucoma, but many pharmaceutical companies are developing their drugs with a different approach in mind. Since these solutions constrict the pupil, ultimately creating a pinhole effect, researchers and clinicians have found the topical therapy useful for the treatment of presbyopia. Now, the presbyopia eye drop market is open, and companies are racing to get their product approved for commercialization.
Here’s a breakdown of what’s available and what’s to come for presbyopia eye drops.
Miotic Agents
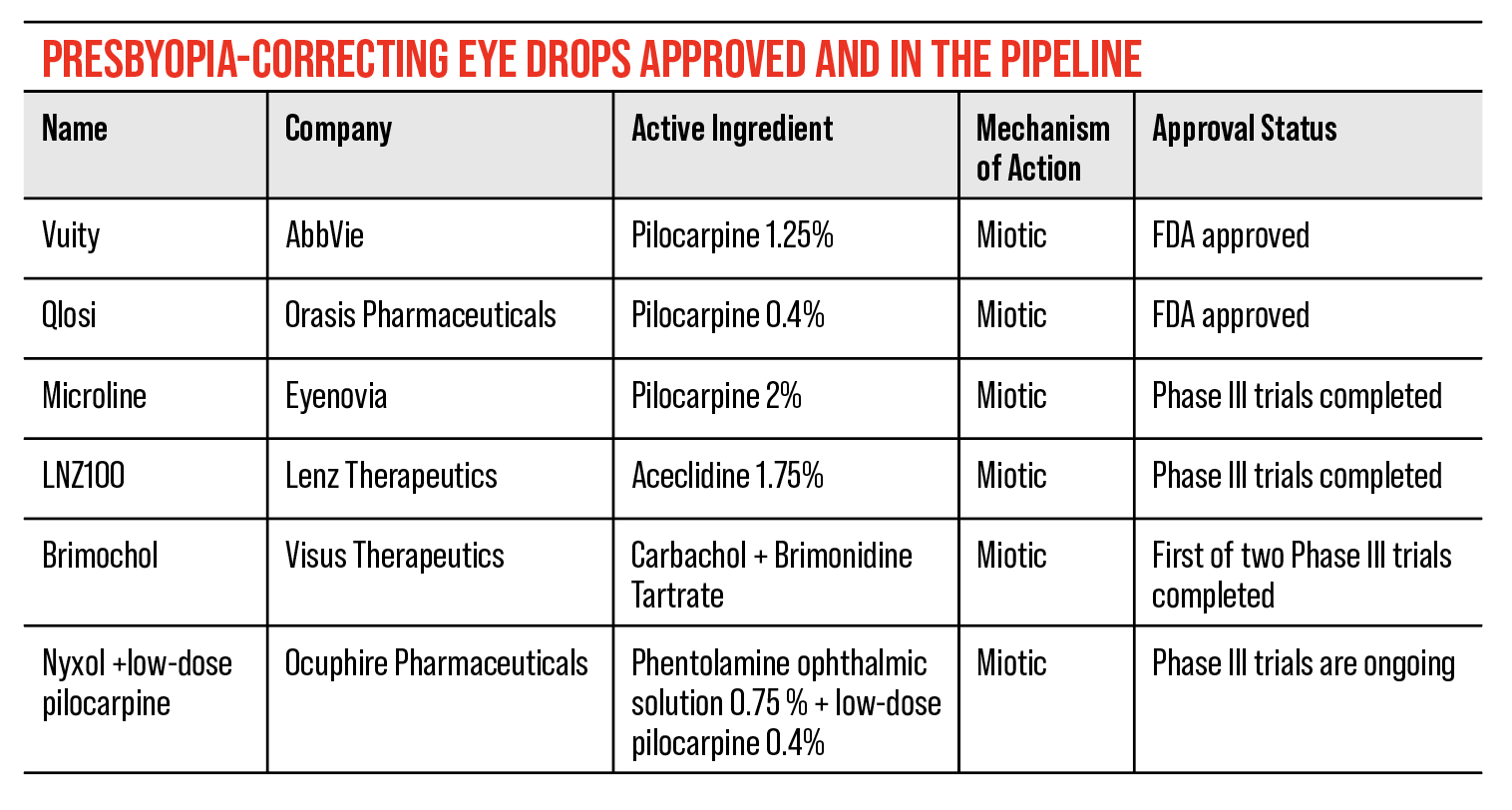
Vuity (AbbVie) was the first presbyopia eye drop to be approved by the U.S. Food and Drug Administration. This is a 1.25% pilocarpine solution, which is one of the miotic solutions commonly used in other presbyopia eye drops. What researchers have found is that the most frequently used miotic agents for glaucoma are essentially the easiest approach for treating presbyopia.
“Pilocarpine and carbachol are the two miotic drops that were most commonly used in glaucoma,” says Richard Lindstrom, MD, the founder of Minnesota Eye Consultants in Minneapolis. “They increased the facility of outflow of the aqueous reducing eye pressure and these drops were showing efficacy in that regard. But there were others as well that were in a different category called acetylcholinesterase inhibitors. Nobody’s really tried to bring those to the market for presbyopia because they’re very powerful. They have a relatively high side effect profile, and they last a very long time, sometimes 24 to 48 hours.”
Besides pilocarpine and carbachol, there are other miotic agents currently under investigation for the treatment of presbyopia. One solution, aceclidine, has been around since the 1960s to treat glaucoma and, at the time, was considered a safer, but slightly weaker, alternative to pilocarpine.1 Another solution, brimonidine tartrate, has been available since 1996 and was approved for managing ocular hypertension and glaucoma.2 It’s also been found in over-the-counter products to reduce redness from minor ocular irritations.
When it comes to using miotics for presbyopia, doctors should consider the concentration of the solution before prescribing it. “The higher the level of concentration of any miotic, the smaller the pupil,” explains Dr. Lindstrom. “And my bias is something in the low twos, or 1.8 to 2.4 mm, as a pupil size that I personally as a clinician think is a good target. You can get even better near vision in a bright light if you go smaller than that. There’s also the possibility of getting longer durability, if you will, meaning longer duration of action with the higher concentrations or with other compounds.”
Interestingly, one off-label indication for miotic solutions is for treating other refractive errors in tandem with presbyopia. “The interesting thing about miotics is that in the bright light, they’re good both for near and distance vision,” states Dr. Lindstrom. “And I think one thing that hasn’t been talked about a lot is the fact that on a bright sunny day, besides helping you see better up close, they also improve your distance vision, right? So, if you have higher order aberrations or if you have low levels of refractive error, maybe a little astigmatism or a little myopia or hyperopia, miotic drops improve your vision.”
Also, miotics have been considered to reduce pupil size in patients who struggle to drive at night. “If you’re a young person wearing contact lenses, which have optical zones in the 6 mm zone or so, if your pupil dilates at night beyond that, then you tend to get halos,” mentions Dr. Lindstrom. “Also with certain intraocular lenses, you sometimes get unwanted visual images at night. Miotic drops can help many of those patients as well by basically reducing the impact of higher-order aberrations and the astigmatism that go along with those situations.”
Latest Market Approval
 |
| For safe use, instruct patients to administer Qlosi at least five minutes apart from other topical ophthalmic medications and avoid contacting the ocular surface with the eye dropper. |
In 2023, the FDA approved Orasis Pharmaceuticals’ low-dose, preservative-free eye drop, Qlosi. Originally CSF-1, this eye drop uses a 0.4% pilocarpine solution for the treatment of presbyopia.
The pooled results from Qlosi’s NEAR Phase III trials demonstrated how the solution is safe and effective for presbyopes.3 Researchers from two Phase III trials enrolled a total of 613 subjects between October 2020 and February 2022. Patients were excluded from the trials if their distance-corrected near visual acuity at 40 cm in one eye wasn’t approximately between 20/50 to 20/160 Snellen at baseline. Each of the subjects, ages 45 to 64, were selected randomly to either receive Qlosi (n=309) or the vehicle (n=304). In order to be successful, the subjects had to achieve at least a three-line gain from baseline in DCNVA at 40 cm without losing one or more lines of CDVA at 4 m on the eighth follow-up day after an hour has passed since their first dose was administered.
According to the results, 40.1 percent of subjects in the Qlosi group achieved the studies’ primary endpoint compared to 19.1 percent of subjects in the vehicle group. When testing each group at different times on day eight of the trials, the researchers discovered that the percentage of subjects who responded positively to Qlosi was significantly more than those in the vehicle group. Subjects from the Qlosi group reported ocular treatmentrelated adverse events such as instillation site pain (5.8 percent), vision blur (3.6 percent) and conjunctival hyperemia (1.6 percent) as well as non-ocular adverse events such as headache (6.8 percent), instillation site/facial pain (1.9 percent; described as brow ache) and nausea (1.3 percent). Additionally, no serious adverse events were reported.
“The most common side effect that we’ve known for decades when you apply a miotic drop is brow ache or headache,” says Dr. Lindstrom. “There are still some patients who can get a brow ache or a headache when they take even the lower concentration pilocarpine drops. Now, what we’ve learned from years using them for the treatment of glaucoma is that if patients use them regularly, then those side effects tend to diminish or even disappear. The other thing we’ve learned, interestingly enough, is that if you just take an aspirin or an Advil 30 minutes before you put the drop in your eye, that tends to reduce the frequency of that type of side effect significantly as well. But the main thing that reduces the frequency is the use of lower concentration of pilocarpine.
“The thing that’s unique about Qlosi is it has a very comforting vehicle as far as the solution that the pilocarpine is placed in,” continues Dr. Lindstrom. “So, the vehicle is basically a topical lubricant. It has hydroxypropyl methylcellulose and sodium hyaluronate, and it’s really comforting when you put it in the eye. The quality topical lubricant tends to enhance your vision rather than reduce it because many patients also have a little bit of a dry eye. In a sense, you’re applying a pilocarpine plus an artificial tear when you put Qlosi in your eye.”
Make sure to explain to patients that Qlosi has been approved for twice-daily administration for up to eight hours of activation. The prescribing information states that it can be administered a second time three to four hours after the original dose. If a patient normally wears contact lenses, they should remove them before administering Qlosi. After 10 minutes, the patient can reinsert their lenses. Qlosi is scheduled to hit the market sometime this year.
 |
Presbyopia Drop Pipeline
Currently, Vuity and Qlosi are the only two FDA approved miotic solutions for the treatment of presbyopia, but there are several others in development in the pipeline.
• LNZ100, LNZ101 (Lenz Therapeutics). In April of this year, Lenz announced positive topline results from its Phase III CLARITY trials for LNZ100 and LNZ101. These topical drugs both use 1.75% aceclidine as the mechanism of action, but LNZ101 incorporates 0.08% brimonidine with the hope of improving the performance of aceclidine on the pupil. After assessing the trial results, Lenz decided to move forward with LNZ100 as a commercial candidate and dropped LNZ101, since it showed similar results to the standard aceclidine solution without improving its performance. They made plans to submit a New Drug Application to the FDA in mid-2024.
The CLARITY 1 and 2 studies examined a total of 698 subjects randomized into a 1:1:1 ration between LNZ100, LNZ101 and a control (CLARITY 1 = 0.08% brimonidine, CLARITY 2 = vehicle). CLARITY 1 had a larger patient population, with a total of 469 accepted subjects. For the studies’ primary endpoint, a significant percentage of subjects would need to achieve a ≥3-line improvement to their near vision assessed over a 10-hour time period. Secondary endpoints recorded were the percentage of subjects achieving at least a two-line improvement to near vision, the mean impact to distance vision when exposed to normal light over time, and the percentage of subjects achieving the primary endpoint on days one, 15 and 28, at 30 minutes, three hours and 10 hours post-dose. Adverse events were also reported.
According to the results, 71 percent of LNZ100 subjects from CLARITY studies achieved the primary endpoint 30 minutes into the trial compared to 12 percent of subjects on the control group. After 10 hours, the longest duration of action, 40 percent of LNZ100 subjects maintained the primary endpoint, while 5 percent of subjects in the control group achieved this goal.
Subjects in the LNZ100 group continued to meet their endpoints throughout the CLARITY studies. One hour after administration, 95 percent of LNZ100 subjects achieved two or more lines of improvement in their near vision and 69 percent of these subjects maintained this throughout the 10-hour time period. LNZ100 subjects’ vision improved by two to four letters of distance vision in normal light without any negative impact. Furthermore, after 28 days, 82 percent of LNZ100 subjects achieved the originally set primary endpoint at 30 minutes, 78 percent of subjects achieved this at three hours and 35 percent of patients achieved this at 10 hours post-dose.
“Aceclidine was used in the glaucoma field and the strength of it, as I look at it, is that it has a longer duration of action,” says Dr. Lindstrom. “So, if you want a drop that lasts longer, then it will last longer with a single drop, and at the concentration Lenz is using makes for an even smaller pupil. In bright light, it’s going to have better near vision.”
The most common adverse events reported across the CLARITY studies were instillation pain (20.1 percent), visual impairment defined as mild dimness (13.2 percent), hyperemia (9 percent) and headache (11.5 percent).
• Brimochol (Visus Therapeutics). In 2023, Visus Therapeutics announced positive topline results from their Phase III BRIO-I trial for Brimochol, a combination of carbachol and brimonidine miotic agents. This is the first drop to show a statistically significant “combination-of-elements” in presbyopia, which is an FDA requirement for fixed-dosed combination products.
According to the BRIO-I study, subjects receiving Brimochol had to achieve at least a 15-letter gain in near visual acuity without a loss of five or more letters at distance. If this was maintained and observed at various time points over a six-hour period, then the primary endpoint would be met. Although a statistical analysis has yet to be published, a total of 182 subjects were administered Brimochol in which 49.4 percent of them achieved the primary endpoint through the 45-day trial.
Brimochol was successful in achieving its secondary endpoints during the BRIO-1 study. There was a statistically significant number of subjects who achieved a 10-letter gain when testing at near, and there was a statistically significant number of subjects whose near visual acuity achieved at least 20/40 Snellen. Additionally, Brimochol subjects gained two letters of distance vision after eight hours post-dose and was statistically significant compared to the results from subjects in the control group.
Some minor adverse events were reported during the study. Eye irritation (14.04 percent) and headache (8.99 percent) were among the most common events. A second Phase III trial, BRIO-II, is underway with more safety and efficacy results possibly coming out this year. This study will observe subjects over a 12-month period and will focus on the long-term effects of Brimochol.
• Nyxol (Ocuphire Pharmaceuticals). Phentolamine Ophthalmic Solution 0.75% is a preservative-free eye drop from Ocuphire Pharmaceuticals. It was FDA approved in 2023 for the treatment of mydriasis under the name Ryzumvi. The company is pushing for the approval of their ophthalmic solution for the treatment of presbyopia, currently titled Nyxol. They’ve completed gathering topline data from their VEGA-2 Phase III trial but haven’t published any results.
The results from the VEGA-1 Phase II trial showed positive results for improving near visual acuity. During the study, a total of 74 subjects were administered a single drop of Nyxol and 73 subjects were administered a placebo. After 12 hours, the subjects’ distance corrected near visual acuity was assessed. The percentage of Nyxol subjects who gained ≥15 letters in DCNVA was 30 percent compared to 14 percent of placebo subjects. More subjects were able to achieve a ≥10-letter gain in DCNVA, with 53 percent of Nyxol subjects achieving this endpoint compared to 28 percent of placebo subjects.
Nyxol subjects experience mild adverse events such as hyperemia, but no headaches or brow aches were reported. Ocuphire has an alternative solution for presbyopes which includes a low dose of pilocarpine along with phentolamine to improve visual acuity further. The addition of pilocarpine didn’t promote any adverse events.
• Microline (Eyenovia). In 2022, Eyenovia announced positive results from their VISION-2 Phase III study of Microline. Since then, not much news has come out about Eyenovia’s decision to move forward with the 2% pilocarpine solution, but some data had been released before then.
 |
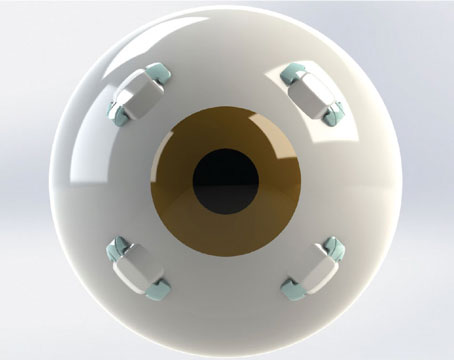
| To ensure presbyopes receive the necessary dosage of Microline, the Optejet is fitted with 109 laser-drilled ports within the spray nozzle which allows for more control over the direction of the spray. Photo: Eyenovia. |
Subjects from the VISION-2 trial were able to achieve at least 15 letters of DCNVA improvement without losing five or more letters of distance vision. Additionally, less than 3 percent of subjects reported some adverse event, which were all mild and/or transient.
Microline is administered using Eyenovia’s Optejet technology, a device that dispenses approximately 8 µL of solution into the eye without the need to tilt the head. This is much smaller compared to the amount of solution dispensed using a traditional eye dropper. This allows the patient to gain the full effect of the solution without wasting any product.
“[Presbyopia eye drops are] new and we’re learning, and we’ve got a lot more to learn, but it’s going to be a positive for the eye-care professional,” says Dr. Lindstrom. “And I would say that means both for the MD and the OD or the physician’s assistant and nurse practitioners who’re seeing these patients.” Currently, 128 million Americans are affected by presbyopia.4 This is nearly 90 percent of the U.S. adult population over the age of 45. The need for presbyopic treatments is growing, which means the need for new treatment options is critical. Having presbyopia eye drops in physicians’ armamentariums will give them the opportunity to satisfy the ever-growing patient population.
“We’ve got another useful tool in our toolbox and we’re going to have more than one option in that toolbox as well with different concentrations and different active pharmaceutical ingredients,” says Dr. Lindstrom. “Over the next five years, we’re going to learn how to help patients benefit from that new modality.”
Dr. Lindstrom is on the Medical Advisory Board and an equity owner of Orasis Pharmaceuticals.
1. Lieberman TW and Leopold IH. The use of aceclydine in the treatment of glaucoma: Its effect on intraocular pressure and facility of aqueous humor outflow as compared to that of pilocarpine. Am J Ophthalmology 1967;64:3:1:405-415.
2. Cantor LB. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother 2000;1:4:815-34.
3. Holland E, Karpecki P, Fingeret M, et al. Efficacy and safety of CSF-1 (0.4%pilocarpine hydrochloride) in presbyopia: Pooled results of the NEAR Phase 3 randomized trials. Clinical Therapeutics 2024;46:2:104-113.
4. New approaches to presbyopia. American Optometric Association. https://www.aoa.org/AOA/Documents/Advocacy/HPI/presbyopia%20brief%20HPI%20Final.pdf Accessed on July 17, 2024.

.png)




