Despite the success of anti-VEGF injections for stabilizing visual acuity and slowing progression in patients with wet age-related macular degeneration, there remains a subset of patients who experience inadequate treatment response. Fortunately, retina specialists have a number of therapeutic options at their disposal when alternative modes of treatment are required.
Here, experts discuss treatment resistance, how they monitor and treat patients who aren’t responding to the initial therapy, and exciting wet AMD therapies on the horizon.
Understanding Treatment Resistance
Resistance in wet AMD may arise from a number of factors, such as genetic predisposition, variation in VEGF expression or disease characteristics. It’s a difficult term to define in the wet AMD space because there isn’t a set of standardized criteria for retina specialists to use when judging treatment response. According to David Almeida, MD, MBA, PhD, of Erie Retinal Surgery and director of clinical research of Erie Retina Research in Pennsylvania, differing interpretations of clinical data and subjective thresholds for inadequate response and true resistance creates variability and, he says, “leaves a lot of vision on the table.”
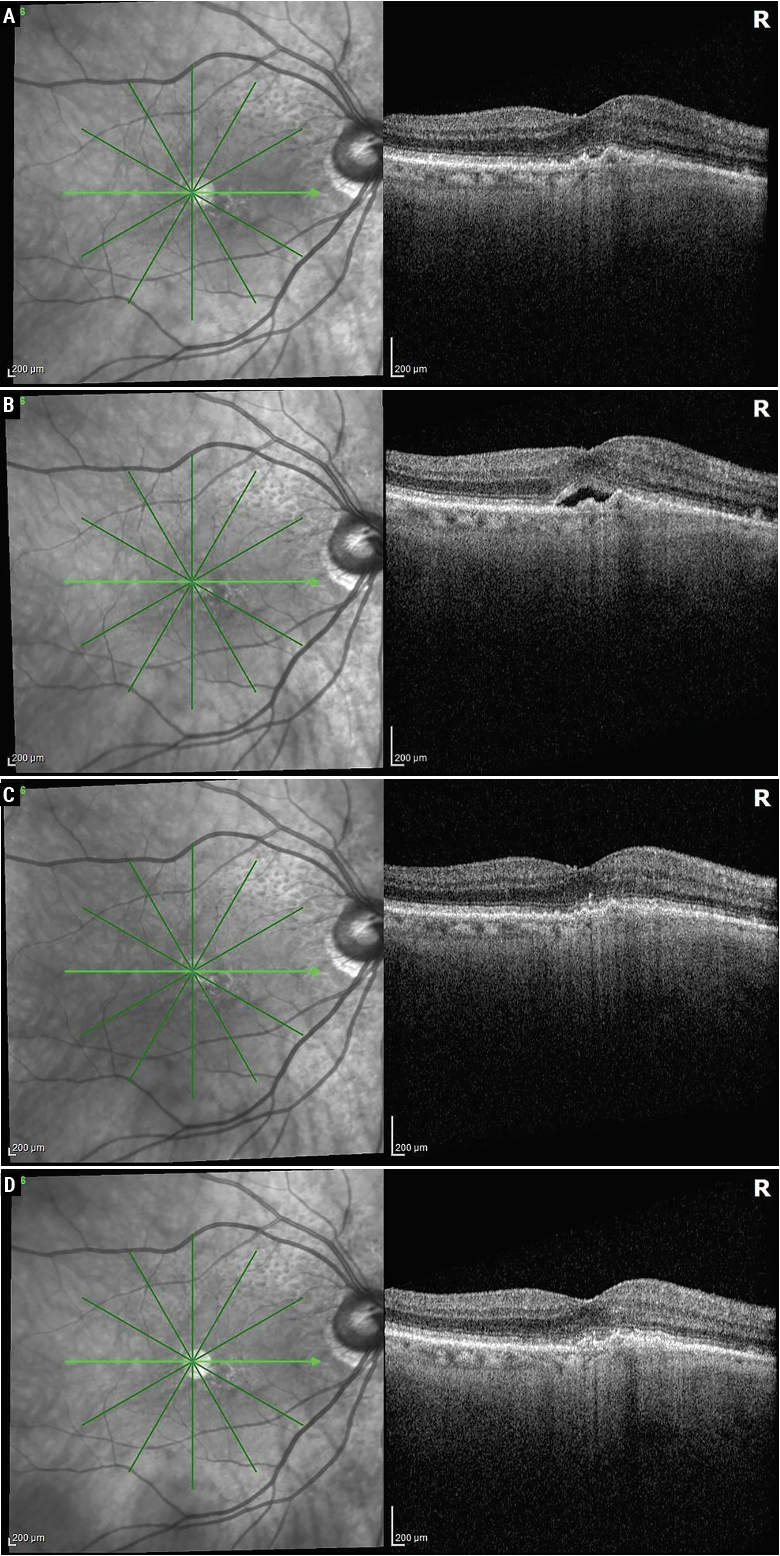 |
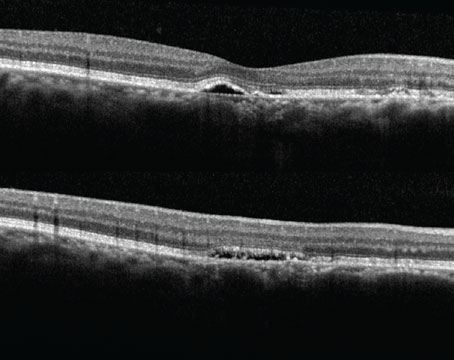
| An 82-year-old patient had a two-year history of exudative AMD in the right eye. She remained essentially fluid-free (A) with an aflibercept 2 mg injection every four weeks but had a decrease in vision and increase in subretinal fluid at five weeks (B). She then received faricimab at four weeks and was fluid-free (C), and she was able to remain fluid-free on faricimab at an eight-week interval (D). Photo: Sunir Garg, MD. |
“The substantial majority of patients respond to treatment, but some patients don’t respond at all, and others don’t respond as well as we’d like them to,” says Sunir Garg, MD, a professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University and an attending surgeon at Wills Eye Hospital in Philadelphia. “As a community of doctors, we’ve struggled with where to draw the line when assessing whether a patient has a complete or incomplete response. What one doctor considers to be a pretty good response, another may view as a glass half empty and call it an inadequate response.”
“Each of us has our own definitions. What we typically do in the clinic is start the patient with a loading dose and assess the response,” says Jay Chhablani, MD, a professor and vitreoretinal surgeon at the University of Pittsburgh School of Medicine and director of clinical research at UPMC Vision Institute. “If they don’t respond after three injections of the same agent, we try to switch drugs. If they don’t respond to that, we may label them as resistant or recalcitrant wet AMD.”
Diagnostics and Biomarkers
When evaluating treatment response, retina specialists employ OCT and assess vision function. “Sometimes I’ll get fluorescein angiography and occasionally indocyanine green angiography. Seldom I’ll get OCT angiography,” Dr. Garg says. “These additional modalities help us to better understand what’s happening in the disease process. They can also be useful to educate the patient in terms of what’s happening, but I don’t often get these additional tests.
“Intraretinal fluid, subretinal fluid and subretinal fibrosis are some of the main things I look for on OCT,” he continues. “I also look at fluctuations in the macular edema. One of the things we’ve learned from recent studies is that patients who have more consistent control of their macula edema tend to do better over the long term, compared to patients who have fluctuations in their edema, where the drug starts to wear off toward the tail end of their treatment interval, then macula thickness increases and then goes back down [with the next injection]. That seems to take a toll on vision as time goes by.”
Dr. Chhablani says, “Some of the research-driven criteria I consider are pigment epithelial detachment, choroidal cleft, sub-RPE fluid and subretinal hyperreflective material or SHRM. Many of these patients present with SHRM, and when we start treatment, it’s important to look at these SHRM characteristics to see whether there’s a good response or not.”
Dr. Almeida adds that “a good prognostic indicator that a patient may have more severe disease is if you can’t make out all of the ellipsoid zone in the macula. Also look for areas of concomitant atrophy with big PED. Big PEDs and atrophy are common causes of rips, which can cause vision loss.”
“It’s important to always ask the patient how they’re doing, because at the end of the day, my goal is to make the patient as happy as I can with their vision,” says Dr. Garg. “Be sure to ask patients about their visual needs and what they’re experiencing. Vision on the eye chart is an imprecise indicator of what the patient’s vision is actually doing.”
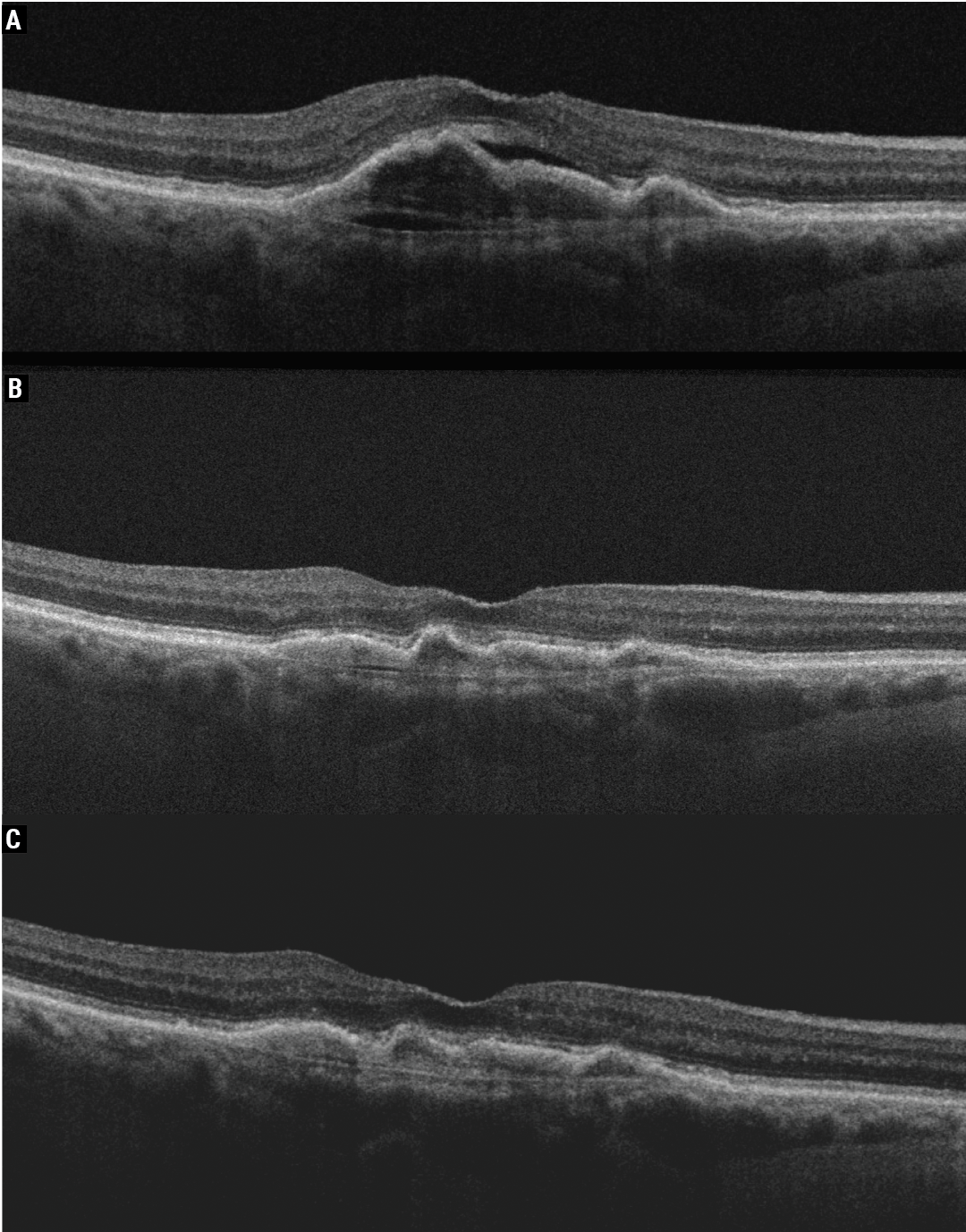 |
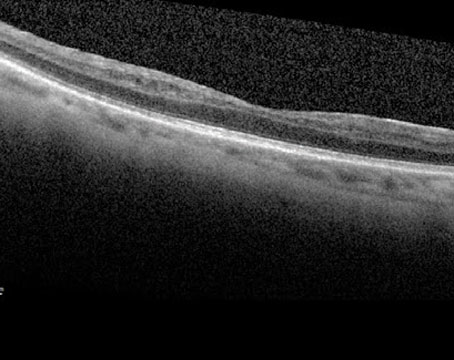
| The right eye of an 85-year-old female patient who had persistent activity with Eylea for wet AMD (A, 20/60) and after undergoing Vabysmo treatment showed a significant reduction in activity (B). The right eye was maintained at six-weekly Vabysmo with no activity (C, 20/30). Photo: Jay Chhablani, MD. |
Management Strategies
Anti-VEGF agents are currently the mainstay of wet AMD treatment. For patients exhibiting treatment resistance, switching drugs or increasing the dosage in certain cases and shortening the treatment interval may get patients back on track.
“There are many options available in our armamentarium for patients who develop resistance to medication, whether it’s a different class of drug or a biosimilar,” says Matt R. Starr, MD, an assistant professor of ophthalmology and vitreoretinal surgeon at the Mayo Clinic. “We usually start with bevacizumab in my practice, and switch to faricimab or aflibercept 8 mg if needed. We’ve also had pretty good success jumping back to something like Cimerli and then trying something different. The Mayo Clinic has a good track record of using biosimilars for oncologic disorders, and we haven’t had any problems. If I start to see a decline in visual acuity or a worsening of fluid, especially intraretinal fluid, those are two reasons I’d want to switch agents.”
“When patients seem to be resistant to treatment, sometimes you just need to do the treatments more frequently, then go back to every four weeks,” Dr. Garg says. “I’ve had a few patients who we’ve brought back in a two-week interval, though that’s not covered by insurance. That can sometimes help to get a better anatomic response and sometimes a better visual response. In other resistant patients, I’ll look to see if they have idiopathic polypoidal choroidal vasculopathy because that can respond well to photodynamic therapy and verteporfin. I’ll also look for PED or subretinal hemorrhage. Typically, I can see those changes on OCT, but sometimes I’ll get ICG angiography. In other patients, we’ll try different drugs. Some patients do better with one particular drug over another.”
The protocols for switching drugs are in part dictated by the step therapy requirements of individual insurance companies. “If I have the luxury of being able to choose drugs for patients, I’ll often start them off with ranibizumab or aflibercept 2 mg, and if I don’t get the response I want, sometimes I’ll switch them to another agent or look into switching to one of the newer drugs,” Dr. Garg continues. “Faricimab and aflibercept 8 mg weren’t looked at for patients who are resistant to treatment; the studies were done on treatment-naïve patients, so we’re not following the Phase III studies when doing this, but I’ve had really good responses. I may not be able to extend their intervals out as much as what patients see on the TV commercials, but even extending it out from five weeks to seven weeks really does alleviate a lot of the treatment burden. It’s great to see.”
Dr. Chhablani says he has a very low threshold for switching drugs since switching in the early stage of the disease often yields a greater response than switching later in the disease stage. “Say I start a patient with a loading dose of bevacizumab,” he says. “I get OCTs monthly. When they come for injections, even in the loading dose, I like to get their OCTs done and I want to evaluate how the response has been, even on one injection. If the response isn’t good after two injections, I’d send the requisition for approval of faricimab, for the dual mechanism, so by the time they come for their fourth injection, they’re already approved and are set to get switched to something else. Considering that faricimab is now approved by more insurances, I tend to switch patients to faricimab more frequently.”
Dr. Chhablani adds that for patients who are doing well with aflibercept 2 mg but aren’t able to extend out beyond four to six weeks, he’ll offer them aflibercept 8 mg to extend them out to eight to 10 weeks.
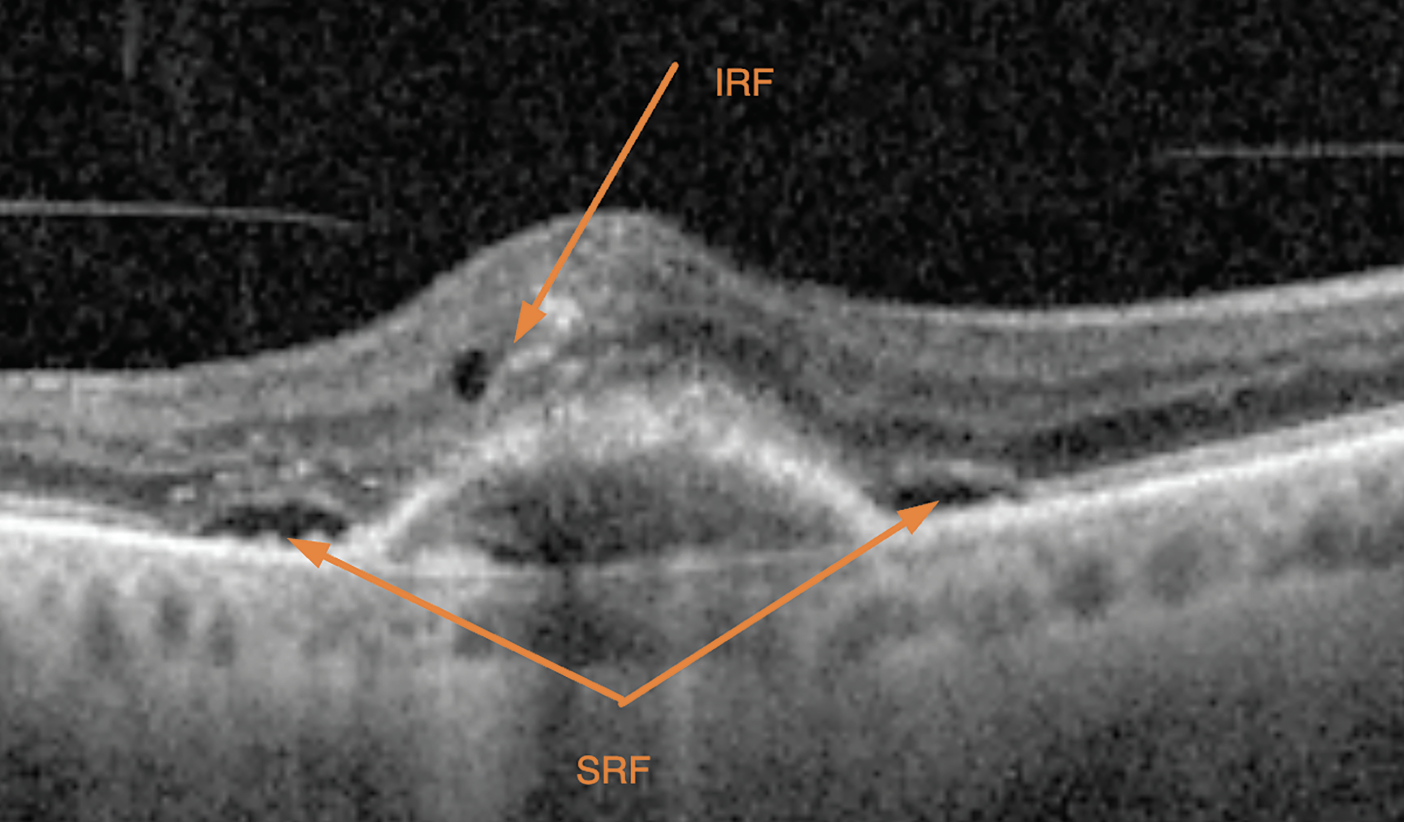 |
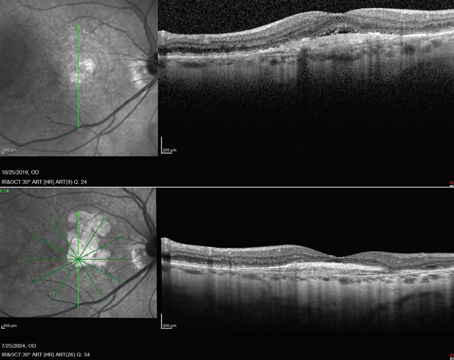
| Intraretinal and subretinal fluid, pictured here, along with other potential findings such as pigment epithelial detachment, choroidal cleft, subretinal fibrosis, sub-RPE fluid or subretinal hyperreflective material, are important indicators of a patient’s disease state and are used to subjectively assess treatment response. Photo: David Almeida, MD, MBA, PhD. |
Combined Therapies
For cases of PCV, combining photodynamic therapy with anti-VEGF may help. “This is the only scenario in which I’d do a combination treatment,” says Dr. Garg, who combines PDT with aflibercept. “I haven’t found combinations of different anti-VEGFs to be helpful clinically to me. I haven’t found that adding steroids to my anti-VEGFs is very helpful either. So, it’s either anti-VEGF monotherapy or occasional additional verteporfin for PCV cases.”
If PCV is suspected, Dr. Chhablani says it’s important to get the patient’s FA-ICG done right away and consider a combination therapy in the early stages of the disease. “In cases where the patient starts on anti-VEGF, is responding and then slowly becomes resistant, get FA-ICG done. There may be a suspicious PCV network,” he says. “For monocular patients who aren’t responding to anything and have no PCV, I’d still consider doing a combination therapy just because sometimes they don’t respond to any anti-VEGF. Doing a combination therapy gives you a double edge to work on. I definitely consider PDT as an option.
“One other combination I use sometimes is a steroid combination if I suspect a massive amount of SHRM or if the PED height is very high and there’s a risk of RPE rip,” he continues. “In those cases, I try to do steroids and anti-VEGF at baseline and then continue anti-VEGF monotherapy. I’ve seen good results with that.”
Tips for Success
Overall, managing treatment intervals, dosage and potential different agents is a balancing act. Here are some key points to keep in mind:
• Reset to the loading dose. “For a patient exhibiting treatment resistance, one thing that works very nicely and gives both of you some time is resetting to a loading dose,” Dr. Almeida says. “Going to a second-generation drug such as aflibercept 8 mg or faricimab and doing two or three loading doses gives you that continuity of seeing the patient every month for two or three months. It gives you time to see how the pathology changes, and it helps the patient understand the idea of teamwork and that you understand what’s important to them. This helps you align the goals of treatment with the patient.”
“Staying engaged with the patient and on top of the disease process is key,” Dr. Garg says. “Sometimes I’ll see patients two weeks after the injection just to better understand what their anatomic response is to the drug, when the drug is at its full strength. Keep trying different things. As long as you have your patient’s best interest at heart and are working to improve their quality of life, the patients will be interested in working with you.”
• Change the method of drug delivery. “If you see early on that a patient is getting worse on a second-generation product, it’s a good idea to engage in a different way in order to prevent the patient from losing more vision,” Dr. Almeida continues. “You have to change the pharmacodynamics of that patient. The port delivery system or Susvimo allows you to go from interrupted drug delivery to continuous drug delivery, which is completely different. This can improve vision over time.” Genentech recently announced Susvimo will be re-introduced at some point this summer.
• Switch the drug class. “Switching to stronger drugs or switching the class of drug is very important,” Dr. Chhablani says. “However, don’t expect faricimab to work as well if you switch the patient after three or four years. Switching earlier produces a better response. Switching the drug class or combining it with steroids or other anti-VEGF can also help.”
• Remain vigilant and diagnose early. “Be aware of any new signs and symptoms,” Dr. Starr says. “Getting patients into the retina clinic sooner can lead to better patient outcomes and better treatment outcomes.”
Dr. Chhablani adds that it’s important to diagnose resistance as soon as possible. “Doing regular scans helps,” he says. “Do the invasive angiography tests. They help us understand the disease profile and the network status, which may reveal things that were hiding such as PCV.”
• Confirm the diagnosis. “If you have a patient who you think should be responding better, go back to the drawing board and investigate whether there’s another diagnosis you’re missing,” Dr. Starr says.
The Future
New approaches on the horizon include gene therapy, sustained-release drug delivery and therapies that address multiple disease pathways. Dr. Almeida and Dr. Chhablani are involved in RegenXbio’s RGX-314 viral vector gene therapy research. They say the results so far are interesting. “This gene therapy is a subretinal therapy which will limit its use to only vitreoretinal surgeons, but I believe the company is exploring suprachoroidal gene therapy as well, and other companies are looking to intravitreal gene therapy,” Dr Chhablani says.
“There’s a lot of interest in tyrosine kinase inhibitors,” Dr. Garg says. “There are nearly half a dozen companies looking at different TKIs, which have an anti-VEGF effect but may also help reduce inflammation in some of these eyes. They have the potential of lasting longer than our current treatments, so that’s exciting, though these treatments are still in Phase I and II.”
“We were all really excited for the port delivery system, it’ll be interesting to see now that it is coming back into the market how it will be used by retina physicians,” Dr. Starr says.
“We’re hopeful that some of the changes with the device design will make [the port delivery system] more consistently good for our patients,” Dr. Garg adds.
Dr. Chhablani says he’s also looking forward to home OCT. “I think that home OCT will help us pick up recurrences much sooner,” he says. “It’ll definitely factor into our treatment plan in the future.”
Dr. Garg is a consultant for Apellis, Bausch + Lomb and Boehringer Ingelheim and performs research for Boehringer Ingelheim, Regeneron, Roche/Genentech, Kodiak Biosciences, Apellis, Iveric Bio, NGM Bio and RegenxBio. Dr. Starr is an advisor for Roche/Genentech, AbbVie/Allergan, Alimera Sciences and Gyroscope Therapeutics. Dr. Chhablani discloses relationships with Eye Point and Iveric Bio. He has stock in Ocular Therapeutix and AbbVie, which owns RegenXBio. Dr. Almeida is a consultant for Roche and Boehringer Ingelheim and is a speaker for Regeneron and Genentech.