Recently, the United States Food and Drug Administration approved Combigan, a fixed combination of brimonidine tartrate 0.2% and timolol maleate 0.5%, making it the first modern combination product to receive approval in the
The Benefits of Fixed Combinations
The concept of fixed combination products that combine multiple medications in a single bottle offers glaucoma patients—and clinicians—some very real benefits. Those benefits include:
• A simpler treatment regimen dramatically increases the likelihood of patient compliance.
• Having to purchase fewer bottles may reduce the cost for patients who are on a copay insurance system.
• If a patient needs two drops at the same time of day, having two medicines in one drop reduces or eliminates the potential washout effect. (A second administered drop, if instilled too soon after the first drop, washes the first drop away before it has a chance to have any pharmacologic impact.)
• Fewer drops in the eye each day means less exposure to potentially harmful preservatives. Preservatives have been implicated in ocular surface disease and may affect subsequent glaucoma filtering surgery by triggering chronic inflammation of the conjunctiva.
• From the clinician's perspective, combination products mean writing one prescription instead of two, going over one set of side effects instead of two, and giving simpler instructions to the patient. With the need for increased efficiency in today's practices, this is a significant advantage.
An Imperfect Set of Options
Of course, the primary reason fixed-combination drugs make so much sense is that many glaucoma patients need more than one eye drop to achieve adequate intraocular pressure control. The Ocular Hypertension Treatment Study showed that 40 percent of patients need more than one drug to achieve a 20-percent reduction in IOP. In patients with established glaucoma we would probably want at least a 25-percent reduction, which would increase the percentage of patients needing adjunctive therapy. And the 40 percent figure only relates to the first five years; we don't have data beyond that point.
For the large proportion of patients who need adjunctive therapy, the choice of an adjunct is challenging. We manage glaucoma today in an era in which we have more IOP-lowering drugs to choose from than ever before. This is wonderful in that it allows us to tailor regimens to individual patients based on their individual needs, but it can be confusing.
When my patients fail to reach their target IOP with a single medication, I use a straightforward decision tree to decide whether to switch or add. If the primary drug lowers IOP poorly—that is, by an amount less than I normally expect from that drug—then I assume the patient responds poorly to that drug, and I switch to something else. But if the primary drug lowers IOP by an amount consistent with my expectations for that drug, and IOP is still above goal, I will continue that drug and add an adjunct.
Given the advantages of having two drugs in a single bottle, it would be ideal to be able to switch these patients to a combination drug that included the current drug plus an adjunct. Unfortunately, to have this option for every patient we'd need to have many more combinations of drugs available.
For many reasons, not every possible pair of glaucoma drugs can be combined in one bottle; many stars must be aligned for a combination to be feasible. For instance, both drugs must be soluble at the same pH or one of the drugs could end up as a clump of powder at the bottom of the bottle. More importantly, the two drugs to be combined must have comparable dosing frequency and timing. This issue may be partly to blame for failure of prostaglandin/beta-blocker combinations to clear the FDA: If beta-blockers work best dosed in the morning, and prostaglandins work best dosed at night, when do you dose the fixed combination?
In balance, there are some downsides to fixed combinations:
• There's no dosing flexibility for individual components. For instance, some Combigan patients might do just as well with once-daily timolol, while others might require three-times-daily brimonidine. In either case, the dosing for some individuals may not be optimized with the fixed combination.
• Drug concentrations are fixed. Combigan contains timolol 0.5%, but some patients might be controlled by timolol 0.25%. This issue also pertains to brimonidine, which is available in various concentrations below 0.2%. In these cases, some clinicians and patients might choose the flexibility of concomitant therapy rather than the rigidity of fixed-combination therapy.
Nevertheless, given the practical advantages of a drug like Combigan, some clinicians may opt to build a simpler regimen using the fixed combination, even if the drugs, dosages and/or concentrations are not individualized for that patient. Whether this kind of potentially less-than-ideal prescription switch will really become a practical issue remains to be seen.
Combigan: The Data
It's worthwhile to summarize the efficacy and safety of Combigan so that clinicians can decide for themselves where it fits into the glaucoma treatment spectrum. The following data is taken from the Phase III trials comparing Combigan to monotherapy with each component1 and comparing it to concomitant therapy with both components,2 as well as from the Combigan prescribing information.
• Combigan lowers IOP more than brimonidine alone or timolol alone at most—but not all—time points. In a 12-month study involving 1,159 subjects,1 the mean decrease from baseline IOP at 12 months was 4.4 to 7.6 mmHg with Combigan dosed twice daily; 2.7 to 5.5 mmHg with brimonidine 0.2% dosed three times daily; and 3.9 to 6.2 mmHg with timolol 0.5% dosed twice daily. (See table, below.) Combigan lowered IOP better than either component alone at 8 a.m., 10 a.m. and 3 p.m.—but at 5 p.m. there was no statistical difference between Combigan and brimonidine monotherapy (because the brimonidine group got its mid-afternoon dose between 3 p.m. and 5 p.m.). The product information supplied with Combigan states that Combigan lowers IOP 1 to 3 mmHg more than brimonidine dosed three times daily and 1 to 2 mmHg more than timolol dosed twice daily.
• Combigan lowers IOP equivalently to, or less than, concomitant therapy with both brimonidine and timolol. In a three-month study involving 371 subjects,2 the difference in IOP between the fixed and unfixed groups was 0.35 mmHg or less at all time points. This study's value is limited by several issues: Concomitant brimonidine was dosed only twice daily (the third dose in the concomitant group would likely have lowered IOP more at some time points in this group compared to the Combigan group); and subjects were enrolled who were already on an IOP-lowering monotherapy at baseline (so absolute IOP change from baseline cannot be accurately determined for either group).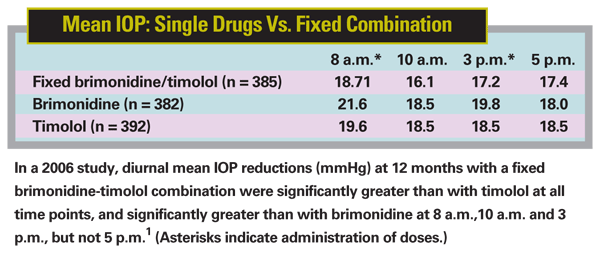
For comparison, the product information supplied with Combigan states that the IOP-lowering efficacy of Combigan dosed as recommended was 1 to 2 mmHg less than the efficacy of the components administered concurrently.
• Combigan's safety profile resembles that of its two components. In the largest Phase III trial, ocular allergy was the most common problem, occurring in 26 percent of Combigan-treated eyes by 12 months.1 Conjunctival hyperemia occurred in 14.5 percent of eyes, stinging in 6 percent and itching in 5 percent. In general, these issues were less common in the Combigan group than in either the brimonidine or timolol monotherapy groups. These adverse events led to discontinuation of Combigan by 14.3 percent of subjects within the first year, compared to 30.6 percent in the brimonidine group and only 5.1 percent in the timolol group.
Clinical Issues
Given the approval of Combigan, several practical questions need to be addressed:
• Where does Combigan fit in the stepped-treatment ladder? Although Combigan's basis for FDA approval consisted of studies evaluating it as primary therapy, it was ultimately approved as adjunctive or second-line therapy. The Combigan product information sheet says that Combigan is indicated "for the reduction of IOP in patients with glaucoma or ocular hypertension who require adjunctive or replacement therapy due to inadequately controlled IOP." This is noteworthy in that the product is indicated for virtually anyone on any IOP-lowering therapy.
Combigan's label does not require that the patient already be using timolol or brimonidine. This means that a patient uncontrolled on prostaglandin monotherapy could have Combigan added to the prostaglandin, and this would be consistent with the label. Such a step would add two drugs at once, rather than making one change at a time, and that would make it difficult to assess the effects of the individual drugs.
• Can we now expect other approvals from the FDA? The approval of Combigan by the FDA may signal that the FDA's stance on fixed-combination products is evolving. In a 2005 interview,3 Wiley A. Chambers, MD, deputy director of the Division of Anti-Infective and Ophthalmology Products at the FDA, stated that "a combination new drug product is potentially approvable if there are studies demonstrating that each of the components that make up the combination contributes in some manner to the safety and efficacy of the drug product."
The key phrase here is "in some manner." In the past, it was believed that this meant a fixed combination must lower IOP by at least 2 mmHg more than either component at all time points. Given the approval of Combigan, the criteria for approval of fixed combination products may be evolving, and that could potentially lead to other fixed combination products being reconsidered in the future.4-7
What's Next?
Enormous amounts of effort and money have been invested into developing fixed combination drugs for the
Dr. Realini is associate professor of ophthalmology at West Virginia University Eye Institute in
1. Sherwood MB, Craven ER, Chou C et al. Twice-daily 0.2% brimonidine-0.5% timolol fixed combination therapy vs. monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: A 12-month randomized trial. Arch Ophthalmol 2006;124:1230-8.
2. Goni FJ; Brimonidine/Timolol Fixed Combination Study Group. 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol 2005:15:581-90.
3. McDermott G. Are fixed combinations worthwhile? Glaucoma Today 2005;3:4:42-45.
4. Rulo AH, Greve EL, Hoyng PF. Additive effect of latanoprost, a prostaglandin F2 alpha analogue, and timolol in patients with elevated intraocular pressure. Br J Ophthalmol 1994;78:899-902.
5. Higginbotham EJ, Feldman R, Stiles M, Dubiner H. Latanoprost and timolol combination therapy vs. monotherapy. One-year randomized trial. Arch Ophthalmol 2002;120:915-922.
6. O'Connor DJ, Martone JF, Mead A. Additive intraocular pressure lowering effect of various medications with latanoprost. Am J Ophthalmol 2002;133:836-837.
7. Feldman RM, Tanna AP, Gross RL et al. Comparison of the ocular hypotensive efficacy of adjunctive brimonidine 0.15% or brinzolamide 1% in combination with travoprost 0.004%. Ophthalmology. 2007;114:7:1248-54.
8. Khouri AS, Realini T,




