In the years since the correction of spherical aberration was introduced in 2002,1 numerous strategies have been advocated for the implementation of this concept. The compelling need to do something was highlighted by a study that found that the contrast sensitivity of pseudophakic individuals was not significantly different from age-matched phakic patients, but was significantly worse than that of younger phakic subjects.2 Thus, although cataract patients had been benefiting (from smaller incisions with phacoemulsification, from advanced instrumentation and techniques for axial length measurement, from appropriate use of advanced intraocular lens power calculations, and from astigmatism management) by attaining uncorrected visions of 20/20 in greater proportions and in shorter postop times, contrast sensitivity was found not to be improved. The need to manipulate spherical aberration derives from the demonstration of the improved clarity of vision (or contrast) under mesopic and photopic conditions, as well as the improved functional performance under night driving conditions.3
In this article, I will review some of the key data as well as proposals that I have put forward to optimize IOL selection while accounting for spherical aberration and other optical factors that affect outcomes after cataract surgery.
Beyond Sphere and Cylinder
In order to understand the strategies, some basic review of the ocular system is required. Wavefront analysis of the ocular optical system has increased our knowledge of the aberrations in the eye, other than sphere and cylinder, which impact visual function significantly. Using Zernike polynomials, the aberrations of the ocular system can be characterized. The Zernike coefficient for spherical aberration has been found to be linked to contrast visual acuity; as this value increases, contrast sensitivity has been found to decrease.4-7 The best contrast sensitivity has been measured in young patients, aged 20 to 30 years.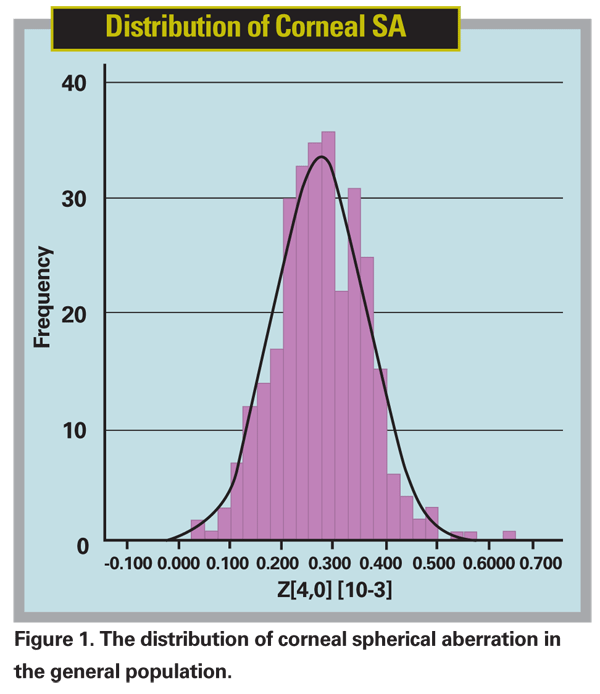
The total higher-order aberrations of the phakic eye are composed of aberrations arising from the anterior corneal surface, the posterior corneal surface, the crystalline lens and the retina. In the aphakic eye, however, 98.2 percent of the aberrations arise from the anterior corneal surface.8 As this discussion is about pseudophakia, then necessarily the corneal aberrations are of importance, and for our purposes, can be thought of being representative of the whole aphakic eye.
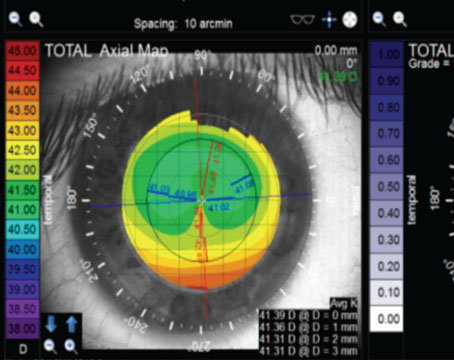
Zernike coefficients of the higher-order aberrations can be derived from corneal topographic data.4,8-11 With current small-incision cataract surgery, it has been reported that the average postoperative corneal topography does not differ significantly from average preoperative corneal topography.12 (Packer M, et al. Customizing selection of aspheric intraocular lenses. Presented at the XXV Congress of the ESCRS, Stockholm 2007) Thus, for cataract patients, it is possible to measure the corneal SAs using corneal topography preoperatively and then use this data to manipulate the outcome of cataract surgery by implantation of aspheric intraocular lenses, with the goal of achieving an optimum SA for the eye and maximum contrast sensitivity.
The cornea has positive SA, which does not vary significantly with aging. The corneal SA has been reported to be approximately +0.27 µm for a diameter of 6 mm.1,13 (Beiko G. Measurement of the wavefront aberrations using the Oculus Easygraph Topographic System. Presented at ASCRS Annual Meeting,
Currently, there are three lenses approved by the Food and Drug Administration for correction of SA. There is the Tecnis (Advanced Medical Optics), the SofPort AO (Bausch & Lomb) and the AcrySof IQ (Alcon Laboratories). The three lenses have different strategies for correction of SA. The Tecnis has a negative SA of 0.27 µm. The SofPort AO lens has an effective SA of almost zero (In fact, the SofPort AO is designed to correct the SA of the isolated lens, as such when it is inserted into the eye; it contributes small amounts of positive SA due to the converging incident light from the cornea.). The AcrySof IQ has a negative SA of -0.20 µm. Thus, if one considers that the average corneal SA of the population is +0.27 µm, then the SofPort AO does not change this value significantly, whereas the Tecnis corrects this SA fully and the AcrySof IQ compensates to a lesser degree.
Strategy: Do Not Use Aspheric IOLs
There have been a small number of studies which have failed to demonstrate any perceived improvement in vision with the use of aspheric IOLs.14-16 (Fry L. Tecnis Z9000 versus Various Other Lenses: Patient Assessment of Vision Quality; ASCRS; San Francisco, April 2003; Fry L. Visual Satisfaction with the SN-60WF Aspheric IOL Versus Conventional IOL; ASCRS; Washington, 2005; Fry L. Subjective Visual Satisfaction With The Alcon AcrySof SN-60WF (aka SN-60 IQ) vs. Conventional SN-60 AT: A Fellow Eye Study; ASCRS; San Francisco, 2006) On this basis, some authors have advocated that there is no need to use aspheric lenses.
However, these reports have some inherent errors. In many, the corneal spherical aberration is not measured. Since the distribution of corneal SA is wide, it is possible that a biased group of individuals was assessed. Similarly, many do not report the pupil size. In order for a measurable effect to be seen, the pupil size has to be greater than 3 mm. (Schmidinger G, et al. Intraindividual Comparison of a Spherical IOL and an Aberration-free IOL. ESCRS,
Another criticism is that patients are assessed with subjective tests, such as the VF 14. These tests are not as sensitive as contrast sensitivity testing and fail to show any improvement. In fact, a standard lens and an aspheric lens can attain similar visual acuity but the aspheric lens will demonstrate superior contrast sensitivity upon testing. (Korogiannis D, et al. Visual Acuity, Contrast Sensitivity and Spherical Aberration Comparison Between Tecnis (aspheric monofocal IOL) and Sensar (AR40e) Intraocular Lenses. ESCRS,
Ocular dominance is also a factor and needs to be studied, as a standard lens in a dominant eye may perform comparably to an aspheric lens in a non-dominant eye. Similarly, the timing of the subjective tests needs to be reported, as neuroadaptation does occur. Although at one month postop, an aspheric lens outperforms a standard lens on contrast sensitivity testing, at three months, the difference in contrast sensitivity testing may be less dissimilar. (Haustermanns A. Quality of Vision with an Aberration Free Aspheric IOL: 1 year data from a Multicentre European Study. ESCRS,
A small or contracted anterior capsulotomy can also negate the effects of an aspheric lens.17 Finally, as will be shown, there is an interaction between spherical aberration and residual refractive error. If this is not controlled, then the visual outcome will be less than ideal.
I would not advocate for standard lenses except in cases in which there is a negative corneal SA, as can occur post hyperopic corneal refractive surgery.
Strategy: Same Aspheric for all Patients
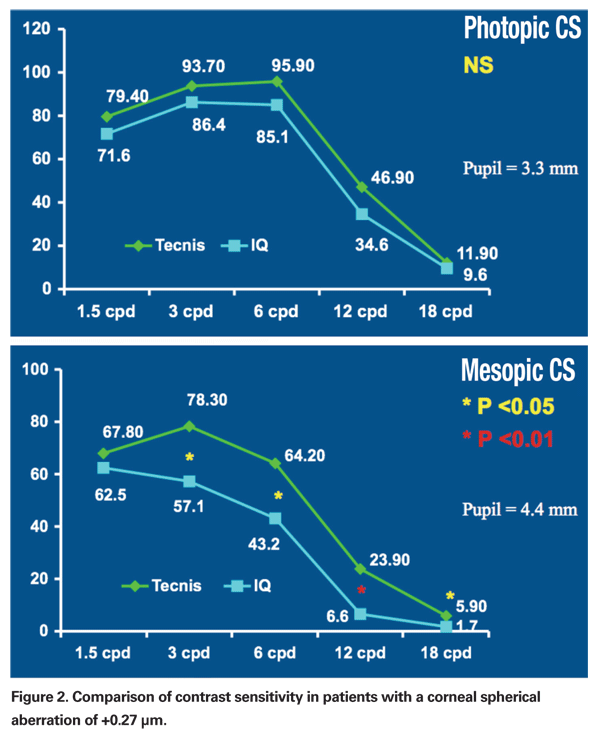
This approach certainly has some evidence to support it. The literature on the AMO Tecnis IOL contains numerous peer-reviewed, prospective, randomized papers that found spherical aberration was reduced or eliminated when a modified, prolate anterior surface IOL is compared to a standard spherical IOL. Many of these studies have found that the functional vision is superior with the Tecnis IOL when contrast sensitivity testing is performed.18-30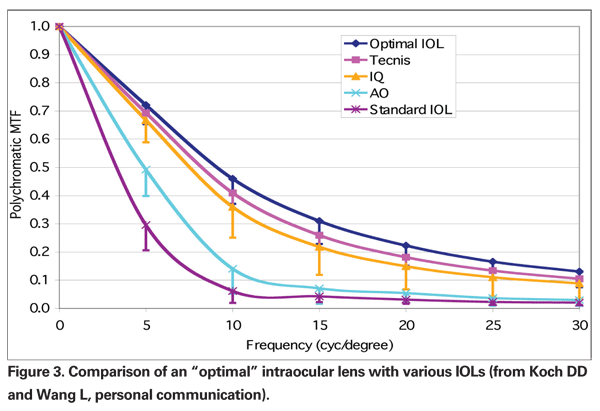
Similarly, studies have also found superior vision with the Acrysof IQ compared to standard lenses.31-34 The Sofport AO has been shown to provide superior visual function to both an aspheric lens and a standard lens when laboratory conditions of decentration are studied.35
The main criticism of the "one lens for all patients" strategy is that it is akin to using a single-powered IOL in all cases of aphakia, irrespective of the parameters of the eye being implanted. This is a good first step, but experience shows that selecting the appropriate power of IOL provides superior uncorrected vision. Similarly, it should not be a huge leap of faith to accept that selecting an appropriate aspheric correction should provide the best functional vision.
Strategy: Target Aspheric Correction
Although the average corneal SA for the population is +0.27 µm, the standard deviation is large and approaches 0.10 µm, or one-third of the value (See Figure 1).13,36 (Beiko G. ASCRS May 2004) The implication of this is that there is a wide spread in the population of the value of the SA and it cannot be assumed that the individual patient undergoing surgery has the "average" value. In fact, there is even further evidence of a racial difference in this value; Asian eyes have been measured to have an average corneal SA of + 0.37 µm. (Nakano EM, et al. Performance of Aspherical versus Spherical Intraocular Lens after Cataract Surgery in Asian Eyes. ESCRS,
This concept of measuring the patient's corneal SA and using an aspheric IOL to target a specific value was tested in 2004. Two groups of patients were studied; the first group was selected on the basis of having a corneal SA of approximately 0.37 µm while the second group was unselected (this group was found to have an average SA of +0.292 µm). Both groups received the Tecnis intraoperatively; thus the first group had a targeted postop value of 0.10 µm while the second group had an average value of 0.00 µm. Postop, photopic and mesopic contrast sensitivity was measured. The group with the targeted value had superior functional vision.
Others researchers have studied this concept. Reports have been presented that support this concept as being workable; measurement of the preop corneal asphericity was followed by implantation of an aspheric lens with a specific target value in mind. Postoperatively, the SA was measured with a wavefront analyzer and found to be near the predicted value. (Packer M, et al.
Thus, based on the data presented earlier, I have advocated measuring the corneal SA preoperatively and targeting 0.10 µm by using the protocol in Table 1 to select the lens to be implanted:
After corneal refractive surgery, some generalizations can be made if one cannot directly measure the SA. Myopic laser ablation tends to increase the SA, so a high-negative SA lens, such as the Tecnis, is recommended. Conversely, hyperopic ablation tends to decrease the SA, and possibly make it negative, so a standard lens with positive SA should be considered.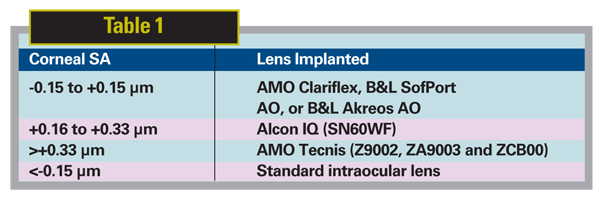
Although I have advocated a target of 0.10 µm, recent studies by Ralph Chu, MD, and by Mark Packer, MD, and colleagues (referenced above) have suggested that targeting 0.00 µm is superior to not targeting any value.
More recently, I undertook a study to determine the ideal target SA. All patients presenting for cataract surgery with a corneal SA of +0.27 µm (at a diameter of 6 mm) were randomized into two groups. One group was implanted with the Tecnis IOL (thus targeting 0.00 µm) and the second to receive the Acrysof IQ lens (thus targeting 0.10 µm). The refractive goal postoperatively was
Unfortunately, since each lens design comes in only one SA power, this targeting approach has limited success. If one is to use a single lens design, then the following strategy has merit.
Strategy: Match Corneal SA and Refraction for Optimization of Vision
It has been known for a while that different optical aberrations interact to affect visual performance.38 However, the nature of this interaction has been elusive.
The first indication of an interaction was reported at ARVO in 2006. (Yoon G, et al. IOVS 2006;47:ARVO E-abstract 59) It was found that patients following corneal refractive surgery preferred 0.25 D of myopia for each +0.10 µm of SA.
More recently, attempts have been made to determine the optimal amount of SA in an IOL. Using data from 154 eyes, Li Wang, MD, PhD, and Douglas Koch, MD, simulated implantation of lenses with different amounts of SA and with different amounts of defocus. They found that the maximum image quality depended on an interaction between the residual SA and the defocus. For plano defocus, a SA of -0.05 µm was found to be ideal; for myopia of -0.5 D, a SA of +0.20 µm; and for hyperopia of +0.5 D, a SA of -0.2 µm was found to give best image quality.39 Interestingly, these calculated values are similar to those reported from clinical experience by the authors of the ARVO study in their corneal refractive paper.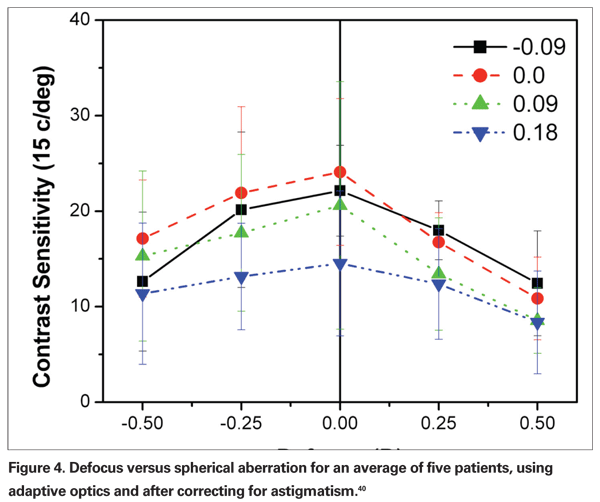
Using the data in the paper quoted above, Drs. Wang and Koch tested the simulated implantation of the optimal IOL against the available aspheric IOLs. They reported that this optimal IOL outperformed the other IOLs in terms of MTF (Koch DD, Wang L. "What is the improvement in optical image quality by custom selection of aspherical IOL?", personal communication. See Figure 3.)
Using adaptive optics to study the optimal spherical aberration for contrast sensitivity, AMO researchers tested different SA against different values of defocus. Figure 4 reveals that the findings mirror those of the previous authors in this section, namely that for an SA of 0.00 µm, maximum vision occurs at 0 defocus; for positive values of SA, a negative defocus provides best vision; and for negative values of SA, a positive defocus was found to be the best.40
These three studies are the basis of a strategy that allows for the interaction of the measured corneal SA (at 6 mm) and a targeted refraction postop so as to optimize vision. But before doing this, one more area needs to be assessed.
In addition to spherical aberration, it is also possible to correct for chromatic aberration. Chromatic aberration impacts negatively on vision and in particular on contrast sensitivity. The chromatic aberration of a material can be expressed in its Abbe number; the higher the number, the lower the chromatic aberration and the greater the optical quality. It has been shown that the AMO acrylic hydrophobic material has the least amount of chromatic aberration of currently used IOL materials (See Figure 5).41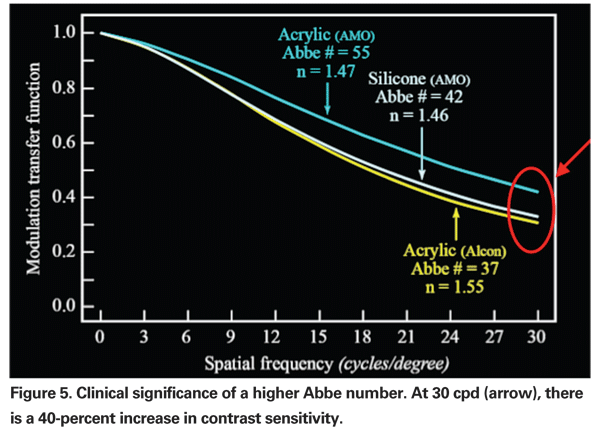
Clinical testing involving different IOL materials has also found that the AMO acrylic material in a standard lens (AMO Sensar) has superior contrast sensitivity compared to the Alcon standard IOL (Acrysof).42 However, the most compelling evidence for the benefits of correcting for both spherical aberration and chromatic aberration was presented at the ARVO 2008 meeting. (Weeber H, et al, IOVS 2008;49:ARVO E-abstract 5275). The research, conducted by AMO R&D, found that a lens which only corrects for spherical aberration improves contrast sensitivity by 20 percent over a standard lens; however, a lens that corrects for both spherical aberration (to 0.00 µm) and chromatic aberration improves visual performance by 50 percent (See Figure 6).
Currently, the lens that corrects for the greatest amount of chromatic aberration and also corrects for spherical aberration is the AMO Tecnis Acrylic IOL. Using the data previously presented to support the concept of targeting a specific postoperative refraction based on the total spherical aberration of the eye so as to optimize vision, I have devised the following protocol: The corneal spherical aberration at 6 mm diameter is measured preop. Since the AMO Tecnis has a SA of -0.27 µm, this value is subtracted from the corneal SA. If the residual value is near 0.00 µm, then a target postop refraction of 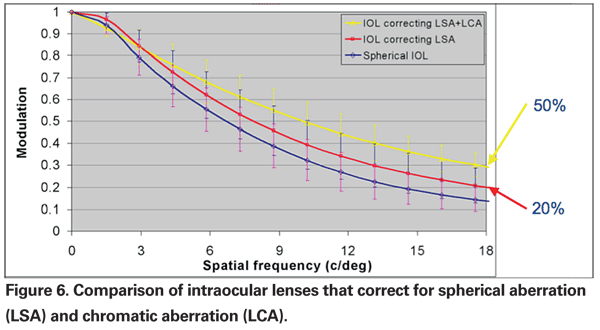
Utilizing this approach of choosing a lens material with the least amount of chromatic aberration, and a lens design that corrects for spherical aberration, with a defocus adjustment to neutralize the SA, would seem to be the best strategy for optimizing vision in cataract or refractive lens exchange patients.
Dr. Beiko is an assistant clinical professor at
1. Holladay JT, Piers PA, Koranyi G, van der Mooren M, Norrby S. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J Refract Surg 2002;18:683-691.
2. Nio YK, Jansonius NM, Fidler V, Geraghty E, et al. Age-related changes of defocus-specific contrast sensitivity in healthy subjects. Ophthalmic Physiol Opt 2000 Jul;20(4):323-34.
3. Tecnis Foldable Ultraviolet Light Absorbing Posterior Chamber IOL (package insert).
4. Guirao A, Artal P. Corneal wave aberration from videokeratography: accuracy and limitations of the procedure. J Optom Soc Am A Opt Image Sci
5. Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res 1999;39:1991-2015.
6. Glasser A, Campbell MC. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res 1998;38:209-229
7. DeValois RL, DeValois KK. Spatial Vision.
8. Barbero S, Marcos S, Merayo-Lloves L, Moreno-Barriuso E. Validation of the estimation of corneal aberrations from videokeratography in keratoconus. J Refract Surg 2002;18:263-270.
9. Rubinowitz YS, McDonnell PJ. Computer assisted corneal topography in keratoconus. Refract Corneal Surg 1989 Nov-Dec;5(6):400-8.
10. Schweigerling J, Greivenkamp JE. Using corneal height maps and polynomial decompensation to determine corneal aberration. Optom Vis Sci 1997;74: 906-916.
11. Gobbe M, Guillan M, Marissa C. Measurement repeatability of corneal aberration. J Refract Surg 2002;18:S567-S571.
12. Guirao A, Tejedor J, Artal P. Corneal aberrations before and after small-incision cataract surgery. Invest Ophthalmol Vis Sci 2004;45:4312-4319.
13. Wang l, Dai E, Koch D, Nathoo A. Optical aberrations of the human anterior cornea. J Cataract Refract Surg 2003;29:1514-1521.
14. Kurz S, Krummenauer F, Thieme H, Dick HB,. Contrast Sensitivity after implantation of a spherical vs. an aspherical intraocular lens in biaxial microincision cataract surgery. J Cataract Refract Surg 2007;33:393-400.
15. Kasper T, Buhren J, Kohnen T. Visual performance of aspherical and spherical intraocular lenses: Intraindividual comparison of visual acuity, contrast sensitivity, and higher-order aberrations. J Cataract Refract Surg 2006;32:2022-2029.
16. The Moorfields IOL Study Group. Binocular implantation of the Tecnis Z9000 or AcrySof MA60AC intraocular lens in routine cataract surgery. Prospective randomized controlled trial comparing VF14 scores. J Cataract Refract Surg 2007:33:1559-1564.
17. Hayashi K, Hayashi H. Effect of anterior capsule contraction on visual function after cataract surgery. J Cataract Refract Surg 2007;33:1936-40.
18. Packer M, Fine IH, Hoffman RS, Piers PA. Prospective randomized trial of an anterior surface modified prolate intraocular lens. J Refract Surg 2002;18:692-6.
19. Bellucci R, Scialdone A, Buratto L et al. Visual acuity and contrast sensitivity comparison between Tecnis and AcrySof SA60AT intraocular lenses: A multicenter randomized study. J Cataract Refract Surg 2005; 31:712-717.
20. Mester U, Dillinger P, Anterist N. Impact of a modified optic design on visual function: Clinical comparative study. J Cataract Refract Surg. 2003;29:652-60.
21. Kershner RM. Retinal image contrast and functional visual performance with aspheric, silicone, and acrylic intraocular lenses. Prospective evaluation. J Cataract Refract Surg 2003;29:1684-94.
22. Kennis H., Huygens M., Callebaut F. Comparing the contrast sensitivity of a modified prolate anterior surface IOL and of two spherical IOLs. Bull Soc Belge Ophtalmol 2004;294:49-58.
23. Martinez Palmer A, Palacin Miranda B, Castilla Cespedes M, et al. Spherical aberration influence in visual function after cataract surgery: prospective randomized trial. Arch Soc Esp Oftalmol 2005;80(2):71-8. Spanish language.
24. Tetz, et. al. Data on File, Advanced Medical Optics, Tecnis Clinical Efficacy Study No. IOLCZ9-0532-005.
25. Mester, Neuhann (2). Data on file, Advanced Medical Optics, Tecnis Optical/Visual Quality Study No. DEV-OPT-0532-002.
26. Packer et al (2). Data on file, Advanced Medical Optics.
Tecnis Visual Quality Study No. DEV-OPT-0532-001.
27. Packer M, Fine HI, Hoffman RS, Piers PA. Improved Functional Vision with a Modified Prolate Intraocular Lens. J Cataract Refract Surg 2004;30:986-992.
28. Ricci F, et al. Low Contrast Visual Acuity in Pseudophakic Patients Implanted with an Anterior Modified Prolate Intraocular Lens. Acta Ophthalmol Scand 2004 Dec; 82(6):718-22.
29. Muñoz G, Albarrán-Diego C, Montés-Micó R, Rodríguez-Galietero A, Alió JL. Spherical Aberration and Contrast Sensitivity after Cataract Surgery with the Tecnis Z9000 Intraocular Lens. J Cataract Refract Surg 2006;32:1320-7.
30. Kasper T, Buhren J, Kohnen T. Visual Performance of Aspherical and Spherical Intraocular Lenses: Intraindividual Comparison of Visual Acuity, Contrast Sensitivity, and Higher Order Aberrations. J Cataract Refract Surg 2006;32:2022-9.
32. Sandoval HP, et al. Comparison of Visual Outcomes, Photopic Contrast Sensitivity, Wavefront Analysis, and Patient Satisfaction Following Cataract Extraction and IOL Implantation: Aspheric vs Spherical Acrylic Lenses. Eye 2007 Jul 6; (Epub ahead of print)
33. Rocha KM, et al. Wavefront Analysis and Contrast Sensitivity of Aspheric and Spherical Introcular Lenses: A Randomized Prospective Study. Am J Ophthalmol 2006;142:750-6.
34. Rocha KM, Soriano ES, Chamon W, Chalita MR, Nosé W. Spherical Aberration and Depth of Focus in Eyes Implanted with Aspheric and Spherical Intraocular Lenses; A Prospective Randomized Study. Ophthalmology 2007;114:2050-4. Epub 2007 Apr 19.
35. Altmann GE et al. Optical Performance of 3 Intraocular lens designs in the Presence of Decentration. J Cataract Refract Surg 2005;31:574-85.
36. Beiko GHH, Haigis W, Steinmueller A. Distribution of the corneal spherical aberration in a comprehensive ophthalmology practice, and can keratometry be predictive of the value of the corneal spherical aberration? J Cataract Refract Surg 2007;33:848-58.
37. Beiko GHH. Personalized correction of spherical aberration in cataract surgery. J Cataract Refract Surg 2007;33:1455-60.
38. Applegate RA, et al. Interaction Between Aberrations to Improve or Reduce Visual Performance. J Cataract Refract Surg 2003;29:1487-1495.
39. Wang L, Koch D. Custom optimization of intraocular lens asphericity. J Cataract Refract Surg 2007;33: 1713-20.
40. Piers PA, Manzanera S, Prieto PM, Gorceix N, Artal P. Use of adaptive optics to determine the optimal ocular spherical aberration. J Cataract Refract Surg 2007;33:1721-6.
41. Zhao H, Mainster MA. The effect of chromatic dispersion on pseudophakic optical performance. Br J Ophthalmol 2007;91:1225-9. Epub 2007 May 2.
42. Kennis H, Huygens M, Callebaut F. Comparing the Contrast Sensitivity of a Modified Prolate Anterior Surface IOL and of Two Spherical IOLs. Bull Soc Belge Ophtalmol 2004;294:49-58.