Mark B. Abelson, MD; Kathryn Kennedy, MSE; and Lauren Lilyestrom, North Andover, Mass.
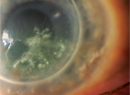
There are few absolutes in the medical world, but endophthalmitis may be an exception: It is the most feared complication of cataract surgery. Bacteria invade the compromised ocular surface and penetrate into the depths of the eye. Symptoms can unexpectedly appear, often around the fourth day, and sometimes after even the most routine and uneventful surgery. Pain and swelling around the eye, light sensitivity and progressive deterioration of vision necessitate an immediate emergency trip to your office to avoid enucleation.
Luckily, endophthalmitis remains a rare affliction, even though retrospective analysis has suggested an increase in the incidence of endophthalmitis in the United States over the past two decades.1,2 These numbers can vary immensely, however, and in some institutions the incidence may actually be decreasing.3 Researchers are scrambling to understand why the condition's prevalence appears to be fluctuating, why it suddenly appears in some patients and what may be done to prevent it. So far, research is inconclusive on the topic of the pathogenesis of endophthalmitis, as multitudes of potential risk factors and an overall low incidence of the condition hamper the discovery of statistically and clinically significant relationships. Here is the latest intelligence on this confounding, though rare, complication.
The Roots
The majority of endophthalmitis cases are caused by gram-positive bacteria, particularly S. epidermidis and S. aureus. Gram-negative bacteria, such as P. aeruginosa, are common offenders in the remaining postoperative cases of endophthalmitis. These organisms are part of the natural eyelid and nasal flora, so conditions such as blepharitis and nasolacrimal duct infections increase the risk of postoperative infections. Systemic infections and diabetes increase the chance of developing endophthalmitis, and immunocompromised patients are also at higher risk.4
Bacteria can also enter the eye through breaches in the sterile field during surgery. Insufficient draping and prep of the surgical area, prolonged and complicated surgeries and inadequate sterilization of surgical tools are all opportunities for bacteria to enter the wound. Postoperatively, delayed healing and wound abnormalities can allow flora into the typically sterile interior of the eye.
The number of potential risk factors associated with endophthalmitis is overwhelming (See Table 1). The recent European Society of Cataract and Refractive Surgery study, conducted in 24 ophthalmology centers throughout several countries, attempted to explore 39 of them. These risk factors ranged from the country in which the surgery was performed to the type of IOL implanted to even the sex of the surgeon.5 A precise and reliable cause-and-effect relationship between any risk factor and the development of endophthalmitis has yet to be identified. For example, leaky wounds and bacteria in the anterior chamber sometimes, but not always,6 lead to endophthalmitis. It's possible that quorum sensing, the ability of bacteria to regulate their virulence in response to various factors,7 could be involved in the varying pathophysiology. Interestingly, wound-edge flora varies daily, and both pathogenic and non-pathogenic bacteria can be recovered from the wound edge without obvious intraocular infection.8 Suffice it to say that virtually any circumstance that increases the chance of bacteria entering the posterior chamber increases the risk of developing postop endophthalmitis.
Explanations for Increases
Usually, new procedures and practices improve upon their predecessors. Why then, does the majority opinion support an increase in endophthalmitis, despite an improvement in hygiene and sterility?
Antibiotic resistance is of particular concern with prophylactic antibiotics. Any exposure of bacteria to antibiotics increases resistance, especially if the dosing regimen is halted or not adhered to. Clinical experience9 and animal models10 have confirmed that antibiotic resistance correlates to more inflammation in and destruction of the retina.
One popularly cited possibility is the increase in sutureless cataract surgeries over the last couple of decades.11 The small incision made in the clear cornea tends to self-heal, has a minimal risk of bleeding and doesn't induce astigmatism. Nevertheless, improper healing can still occur, transforming the wound into a gateway for bacterial colonization.12 Furthermore, fluctuating intraocular pressure can cause the incision to periodically pop open and close. In general, higher IOPs are associated with more tightly sealed incisions; however, the incision angle11,13 (which can be influenced by something as simple as surgeon handedness14) can alter this relationship.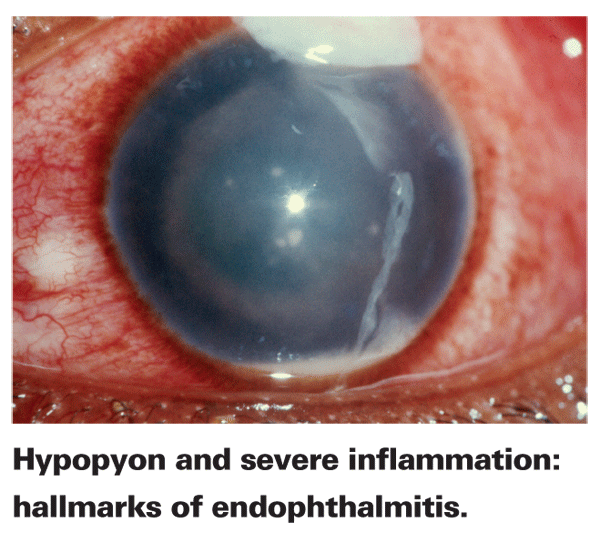
Popular opinion and several studies implicate clear corneal incisions as a prime reason for increased incidence of endophthalmitis.11 Of course, conflicting studies also exist.7 Given the overall low incidence of endophthalmitis, studies are plagued by statistical concerns. These, combined with logistical concerns (e.g., the inability to control for other potential risk factors15) cast doubt on any direct and absolute cause-and-effect relationship.
The ESCRS study,5 like many endophthalmitis studies, encountered many variables that complicated data analysis. The response variable was binary, with only 29 (20 confirmed) cases of endophthalmitis occurring in more than 16,000 patients. Excluding age, all the variables in the model were categorical, each with either two or three levels, resulting in 192 possible combinations of the factors. Twenty-nine (or 20) observations spread over 192 categories means that the majority of the categories have few, if any, observations, which makes definitive relationships difficult to find.
One relationship, however, that has been garnering a lot of attention is that of prophylactic intracameral injections and a corresponding reduction of postoperative endophthalmitis.
Intracameral Injections
Various combinations of pre-, intra- and postoperative antibiotics are typically used as prophylactic measures against endophthalmitis. Protocol as to which regimen is used varies with ophthalmologist preference, the condition of the individual patient, policies at a given practice, etc.16 Although drops or systemic antibiotics have been traditionally used, intracameral injections have been attracting attention as a possible "magic bullet" for the prevention and treatment of endophthalmitis. Intracameral injection describes prophylactic injections into the anterior chamber, or injections into the vitreous when a patient presents with endophthalmitis. The idea is to reduce the flow of bacteria from the anterior into the posterior chamber by increasing the amount of antibiotic within the anterior chamber.
The ESCRS study concluded that intracameral cefuroxime administered prophylactically at the end of surgery significantly decreased the risk of endophthalmitis.5 Three of the proven endophthalmitis cases had received intracameral injections of cefuroxime (likelihood ratio test p=0.001). Since the subjects were randomized to detect differences in outcomes between the two prophylactic measures, these results appear to be fairly conclusive.
Fourth-generation fluoroquinolones have a broader spectrum and greater penetration into the ocular tissues than older antibiotics, and are being investigated for use in intracameral injections.17 Although the anatomy of the rabbit eye differs considerably from that of the human eye, investigations have demonstrated safety and efficacy with moxifloxacin 0.5% injected into the vitreous cavity of rabbits.17 Preliminary clinical investigations have echoed this finding.18
Some European countries regularly use prophylactic intracameral injections of cefuroxime, and studies have found significant correlations between the injections and reduced incidence of postoperative endophthalmitis.16 It's worthwhile to also note that pre- and postoperative topical antibiotics are not the standard of care in Europe, and don't significantly reduce the incidence of endophthalmitis.19 The ability of a topical antibiotic to prevent endophthalmitis is largely determined by its ability to penetrate the anterior chamber and kill bacteria before it can reach the vitreous.4 This is a huge task for any antibiotic. Topical treatment, therefore, can't be relied upon for preventing endophthalmitis.
What Should Be Done
The myriad opportunities for bacteria to enter the incision transform the obligatory ounce of prevention into pounds of astute awareness of sterile technique. Unfortunately, the low incidence of endophthalmitis makes assessing the effectiveness of preventive techniques difficult and inconclusive. The standard prophylactic regimen varies. Guidelines of the
Since many cases of endophthalmitis are believed caused by the patient's own lid flora, proper draping and preparation of the surgical area is crucial. The application of 5 to 10% povidone-iodine solution prior to surgery is considered mandatory by all three of the aforementioned organizations. Application of povidone-iodine is a standard procedure in the
Any antibiotic regimen that is used prophylactically (such as intracameral injections) must have a minute risk of adverse effects, ideally less than the risk of otherwise developing endophthalmitis. Topically applied fourth-generation fluoroquinolones are favored by 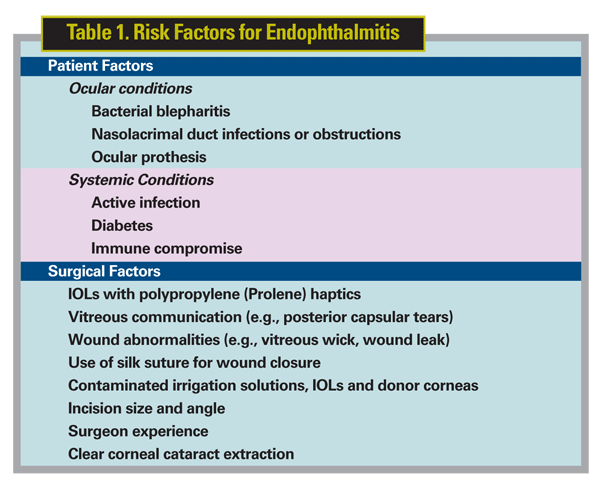
When endophthalmitis develops, the effectiveness of treatment is dependent on both the severity of the infection and on the elapsed time between the appearance of symptoms and treatment. Initial symptoms of developing endophthalmitis include ocular discomfort, lid swelling and a deep red eye. Increased anterior chamber reactions—particularly cell and flare, fibrin and hypopyon—further signify the onset of endophthalmitis. Primary treatment involves any combination of topical, systemic and intravitreal antibiotics and careful monitoring. Steroids may also be used to reduce inflammation. Severe cases may require a vitrectomy; the worst cases ultimately result in enucleation.
Although the risk of endophthalmitis is considered to be minimal, the visual outcomes for individuals who develop this intraocular infection can be devastating. Proper sterile technique, including the preop use of povidone-iodine, can control the amount of bacterial flora on the patient's ocular surface during surgery, and therefore must be used. Intracameral injections of antibiotics may offer effective prophylaxis for at-risk patients. Ultimately, the need for surgery and prophylactic therapies and risk of developing endophthalmitis must be assessed and balanced for each patient in order to guarantee the best visual outcomes. Endophthalmitis is essentially a random occurrence, and all surgery patients have some risk of developing postoperative infections. A magic bullet against the development and for the treatment of endophthalmitis isn't available, and isn't likely anytime soon.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol 2005;123:5:613-20.
2. West ES, Behrens A, McDonnell PJ, et al. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology 2005;112:1388-94.
3. Eifrig CW, Flynn HW Jr, Scott IU, Newton J. Acute-onset postoperative endophthalmitis: Review of incidence and visual outcomes (1995-2001). Ophthalmalic Surg Lasers 2002;33:5:373-8.
4. Ng EwM, D'Amico DJ. Postoperative Endophthalmitis. In Albert DM, Jakobiec FA, eds. Principles and Practice of Ophthalmology.
5. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007;33:978-88.
6. Egger SF, Huber-Spitzy V, Skorpik C, et al. Different techniques of extracapsular cataract extraction: Bacterial contamination during surgery. Prospective study on 203 consecutive patients. Graefes Arch Clin Exp Ophthalmol 1994;232:5:308-11.
7. Schauder S. The languages of bacteria. Genes Dev 2001;15:12:1468.
8. Abelson MB, Allansmith MR. Normal conjunctival wound edge flora of patients undergoing uncomplicated cataract extraction. American Journal of Ophthalmology 1973;76:4:561-5.
9. Dorey MW, Ford BA, George SP. A study of postoperative endophthalmitis in
10. Miño de Kasper H, Hoepfner AS, Engelbert M, et al. Antibiotic resistance pattern and visual outcome in experimentally-induced Staphylococcus epidermidis endophthalmitis in a rabbit model. Ophthalmology 2001;108:3:470-8.
11. Taban M, Rao B, Reznik J, et al. Dynamic morphology of sutureless cataract wounds—effect of incision angle and location. Survey of Ophthalmology 2004;49:S62-S72.
12. Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson RJ. Cohort study of 27 cases of endophthalmitis. J Cataract Refract Surg 2005;31:4:735-41.
13. Humayun M, Gottleib CC,
14. Miller JJ, Scott IU, Flynn HW Jr, et al. Acute-onset endophthalmitis after cataract surgery (2000-2004): Incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol 2005;139:983-7.
15. Schein OD. Prevention of endophthalmitis after cataract surgery: Making the most of the evidence. Editorial. Ophthalmology 2007;114:5:831-2.
16. Gordon-Bennet P, Karas A, Fianagan D, Stephenson C, Hingorani M. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the
17. Kowalski RP, Romanowski EG, Mah FS, Yates KA, Gordon J. Intracameral Vigamox (Moxifloxacin 0.5%) is non-toxic and effective in preventing endophthalmitis in a rabbit model. Ophthalmol 2005;140:497-504.
18. Espiritu CR, Capaas VL, Bolinao JG. Safety of prophylactic moxifloxacin 0.5% ophthalmic solution in cataract surgery patients. J Cataract Refract Surg 2007;33:1:63-8.
19. Barry P, Seal DV, Gettinby G, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refr Surg 2006;32:3:407.
20. Cataract in the Adult Eye.
Cataract_in_the_Adult_Eye.pdf. Accessed Oct. 24 2007.
21. Managing an Outbreak of Endophthalmitis. The
22. Trinavarat A, Atchaneeyasakul LO, Nopmaneejumruslers C, Inson K. Reduction of endophthalmitis rate after cataract surgery with preoperative 5% povidone-iodine. Dermatology 2006;212:35-40.
23. Chang DF, Braga-Mele R, Mamalis N, et al. Prophylaxis of postoperative endophthalmitis after cataract surgery. Results of the 2007 ASCRS member survey. J Cataract Refract Surgery 2007;33:10:1801-5.