Despite remarkable advances in our ability to treat glaucoma over the past few decades, it remains a devastating problem in many parts of the world. One reason for that is that our surgical treatment options tend to be challenging to perform, costly and involve significant risks of long-term complications. In many parts of the world, these factors help to make effective treatment difficult at best.
In a recent presentation at the 13th annual Yale Glaucoma Symposium, M. Bruce Shields, MD, Marvin L. Sears Professor and Chairman Emeritus of Ophthalmology & Visual Science at Yale University School of Medicine, spoke about past and current approaches to enhancing aqueous outflow, including his ongoing work with enhancement of the eye's uveoscleral outflow pathway.
"The reality is that the glaucoma surgical options we've been relying on for the past 100 years don't really meet the criteria we'd like to see," says Dr. Shields. "As a result, there's been a lot of research devoted to developing new glaucoma procedures, especially in the past decade. Several new alternatives to traditional techniques are being evaluated or are in use right now. Notably, while many traditional options create a pathway for aqueous to flow out of the anterior chamber into a subconjunctival space—which is not a physiologic approach—several of the newer options are striving to enhance the existing physiologic outflow pathways."
Four Roads to Travel
Dr. Shields finds it useful to divide approaches to enhancing outflow into four categories. "One group of options attempts to enhance outflow through the trabecular meshwork and Schlemm's canal," he says. "Alternatives such as canaloplasty, in which a suture inside Schlemm's canal is used to provide tension to hold the canal open, and the Trabectome, a handheld instrument that uses microelectrocautery to ablate a strip of tissue from the trabecular meshwork and Schlemm's canal, allowing the aqueous direct access to the outflow pathway, fall into this category. This approach is where most of the action and money are right now. However, this kind of approach often fails to lower pressure sufficiently; with the Trabectome, clinical data has shown mean postop pressures to be around 16 mmHg;1 with canaloplasty, postop pressures have been around 13 or 14 mmHg.2 In some patients, we really need to get pressures lower than this.
"A second category of outflow enhancement aims to direct the aqueous humor outside of the eye, onto the cornea, or at the limbus, or even back into the cul-de-sac," he continues. "Devices in this category include the transcorneal shunt. What prevents endophthalmitis, in theory, is the presence of millipore filters that allow the aqueous to egress, but prevent microorganisms from entering the eye. Several teams are exploring this type of outflow strategy."
Dr. Shields's third category includes traditional approaches such as trabeculectomy that direct aqueous humor into a subconjunctival space. "This creates a reservoir of fluid that raises the conjunctiva, creating a so-called filtering bleb," he notes. "The aqueous humor literally filters across the conjunctiva and is washed away in the tear film. Drainage implant devices such as the Baerveldt and Ahmed tube shunts also fall into this category."
|
"Given that fact," he adds, "it's worth considering whether drainage implant devices that shuttle fluid into the subconjunctival space are really that much better than trabeculectomy. They too have their potential problems: a complicated postoperative course; hypotony or hypertension; corneal decompensation and tissue erosion; and they often fail to lower intraocular pressure as far as we'd like."
Dr. Shields's fourth category includes approaches that attempt to enhance flow through the uveoscleral outflow pathway, another physiologic route. "Some drugs, such as the prostaglandins, appear to work by increasing flow through this pathway," he notes. "The better-known surgical approaches in this category include cyclodialysis and implantation of SOLX's gold microshunt.
"To my mind, when searching for better glaucoma treatments it makes sense to try to enhance the physiologic routes, thus hopefully avoiding many of the potential complications associated with the nonphysiologic routes," he says. "For that reason, my own focus has largely been on developing a device that can enhance uveoscleral outflow—and do so in a way that will be safe, simple and cost-effective enough to allow widespread glaucoma treatment in developing countries around the world."
The Uveoscleral Pathway
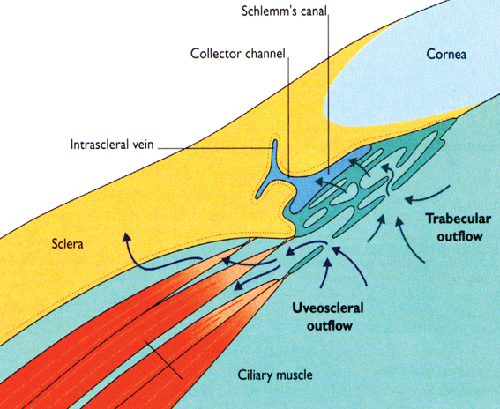
Dr. Shields explains that the uveoscleral outflow route can be thought of as consisting of two segments—the uveal portion and the scleral portion—through which aqueous makes its way during its journey out of the eye. The uveal portion consists of the area containing the longitudinal muscle bundles of the ciliary body; aqueous flows from the anterior chamber through this region to reach the suprachoroidal space. The scleral portion goes from the suprachoroidal space through the sclera and into the lymphatics in the orbit outside of the eye.
"The reason it's important to think of these segments separately is that they affect the flow in different ways," he says. "The uveal segment is the rate-limiting part of the pathway. Resistance to uveoscleral outflow occurs primarily here, most likely within the muscular portion of the ciliary body. In contrast, once fluid reaches the suprachoroidal space, there's little resistance to flow; structurally, there's really nothing to slow down the movement of fluid.
"What this means," he continues, "is that the uveal portion is relatively pressure-independent, so if the eye has not been altered by surgery or trauma, raising the pressure inside the eye will not increase the pressure in the suprachoroidal space proportionately. For example, one study using a monkey model found that raising the intraocular pressure from 10 to 60 mmHg only caused the pressure differential between the anterior chamber and suprachoroidal space to rise from 3 to 10 mmHg.3
"This also accounts for one of the big problems with cyclodialysis, in which the surgeon creates a direct opening from the anterior chamber to the suprachoroidal space," he notes. "Removing part of the rate-limiting barrier increases the likelihood of hypotony.
Although cyclodialysis was often used during the first half of the 20th century, it eventually fell out of popularity because of the complications associated with it. Those complications include the possibility of the pathway created between the anterior chamber and the suprachoroidal space suddenly scarring closed, causing IOP to shoot sky-high. Given these issues with cyclodialysis, recent attempts to increase outflow through the uveoscleral pathway have focused on insertion of a shunt that can more successfully regulate the flow and maintain the patency of the opening."
Dr. Shields points out that at one time, the percentage of outflow occurring via the uveoscleral pathway in human eyes was thought to be small—between 5 and 15 percent of total aqueous outflow. However, researchers eventually realized that this data came from studies involving older eyes; further research revealed that young human eyes have a much greater percentage of outflow occurring through the uveoscleral pathway—perhaps as much as 40 percent. "Changes in these tissues as we age cause the flow to decrease—which may explain the different observations among investigators regarding the contribution of the uveoscleral pathway to physiologic outflow," he says.
"There are several factors known to produce an increase in uveoscleral outflow," he continues. "The prostaglandins that we use today work primarily through this pathway.
They appear to stimulate a group of enzymes called matrix metalloproteinases that break down some of the fibrous tissue connecting the longitudinal muscle bundles in the ciliary body. This opens up the space somewhat, allowing the aqueous to flow out more easily." (The effect on this tissue is apparently temporary, since IOP rises again once prostaglandin drops are stopped.)
Dr. Shields notes another factor that increases uveoscleral outflow: low-grade inflammation.4 "In fact, prostaglandins may actually create low-level inflammation; we know they create inflammation at higher levels," he says. "This may be one reason why overuse of prostaglandins can be counterproductive; higher levels of inflammation can cause swelling of the endothelial cells in the trabecular meshwork or cause protein to build up, clogging the outflow through that pathway and offsetting any beneficial effect on the uveoscleral pathway. In addition, inflammation can affect aqueous production. So prostaglandins can raise or lower the pressure, depending on the relative balance between their effect on uveoscleral outflow, trabecular outflow and aqueous production, all of which seem to be dose-dependent. It's a very complex balance that calls for a very specific level of prostaglandin use."
Creating a Uveoscleral Shunt
"Realizing that we needed something better than cyclodialysis to enhance uveoscleral outflow, surgeons began looking at the possibility of placing a device in the tissue between the anterior chamber and the suprachoroidal space," says Dr. Shields. "A number of alternatives have been tried over the years. Early devices were made of solid materials, which weren't effective, so subsequent research turned to tubes. The one that's been researched the most in recent years is the gold microshunt from SOLX. The device contains a number of very small parallel channels, some of which are open and some of which are closed; the idea was that a laser could open up extra channels as needed to maintain flow, making the device titratable. Research using the device is ongoing; the microshunt appears to be effective, but may not lower pressure as much as some patients need5—a problem shared by subconjunctival shunts such as the Ahmed and Baerveldt."
|
"At this point we have 15 patients at two clinical sites with follow-up of six to 12 months," he continues. "A few patients in early testing have achieved pressures in the low teens or even single digits, but one or two patients have been outright failures.
Overall, the results so far have not been as good as we would like. However, the Aquashunt is still early in its development, so we expect problems and setbacks; we're going to learn from these and find ways to improve the device, as well as our insertion techniques."
Dr. Shields notes that the initial trials revealed more of a tendency for fibrosis or scarring in the suprachoroidal space than his team had hoped. "We've seen this in both our rabbit and initial human trials, and it may account for the few cases in which the Aquashunt hasn't worked," he says. "Of course, the uveoscleral route is a physiologic pathway in which fibrosis would not normally be a concern. For that reason we hypothesize that putting a foreign body into that space could be stimulating the fibrosis.
We're now working with colleagues at Yale to see whether it's possible to modify fibrosis in the suprachoroidal space pharmacologically. Antifibrotic agents could be released from the lumen and wash back into the space to prevent scarring, for example. We're also investigating the possibility of options such as putting a coating around the device to minimize scarring."
Aiming for a Global Solution
Dr. Shields hopes that the Aquashunt will ultimately be an effective and easy-to-use device. "We want to offer doctors a treatment that enhances an existing physiologic outflow pathway; one that's quick and simple to insert so that many people can use it; and one that has predictable outcomes with minimal postop complications," he says. "Such an option could be a major advance in our ability to help people not only at home but in parts of the world where, today, glaucoma is almost a hopeless condition." REVIEW
1. Minckler D, Baerveldt G, Ramirez MA, Mosaed S, Wilson R, Shaarawy T, Zack B, Dustin L, Francis B. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc 2006;104:40-50.
2. Shingleton B, Tetz M, Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one-year results. J Cataract Refract Surg 2008;34:3:433-40.
3. Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci 1989;30:2:233-8.
4. Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci 1987;28:3:477-81.
5. Melamed S, Simon GJB, Goldenfeld M, Simon G. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: A pilot study. Arch Ophthalmol 2009;127:3:264-9.