Thirty-four eyes of 17 patients (mean 46.35 years ±11 [SD]) complaining of persistent dryness more than 12 months after LASIK were prospectively studied by symptom scoring and kinetic analysis of tear interference image, tear breakup time, and fluorescein clearance test. Once patients were clear of inflammation and treated for aqueous tear deficiency, lipid tear deficiency was further confirmed and treated with Eyefeel (Kao Inc.), an eye-warming device, four times daily for four weeks.
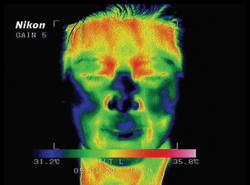 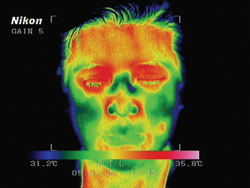 |
| Infrared photos show the temperature rise before (left) and five minutes after the use of the Eyefeel device. Reprinted with permission from J Cataract Refr Surg. Elsevier |
Sixteen patients were asymptomatic before LASIK, but dryness persisted for 41 ±19.3 months. Delayed tear clearance was observed in 15 patients (88.2 percent) and floppy lids in 12 patients (70.5 percent). Aqueous tear deficiency was reconfirmed in 16 eyes (53.3 percent). After Eyefeel treatment, there was a subjective improvement of ocular surface diseases index from 60.6 ±10.6 to 25.8 ±18.5 (p=.0007). Tear breakup time was improved from 2.4 ±3.9 seconds to 7.9 ±3.6 seconds (p=.004). There was a tear interference pattern change from a vertical lipid tear deficiency to a horizontal normal in seven eyes. There was a mean lipid spread time improvement from 1.3 ±0.4 sec to 0.8 ±0.4 sec (p=.001), and there was a mean lipid thickness improvement from 63.5 ±23 nm to 79.5 ±27 nm (p=.04).
(J Cataract Refract Surg 2005;31:1741-1749)
Di Pascuale M, MD, Liu T-S, Trattler W, Tseng S.
UHR OCT Superior to Standard
Ultrahigh-resolution Optical Coherence Tomography enhances the visualization of intraretinal architectural morphology relative to standard-resolution OCT, says a group at Massachusetts Institute of Technology. UHR OCT images can provide a baseline for defining the interpretation of standard-resolution images, enhancing the clinical utility of standard OCT imaging. In addition, UHR OCT can provide additional information on macular disease morphology that promises to improve understanding of disease progression and management, the group concludes.
The research sought to compare UHR OCT with standard-resolution OCT for imaging macular diseases, develop baselines for interpreting OCT images, and identify situations where UHR OCT can provide additional information on disease morphology. The cross-sectional study involved 1,002 eyes of 555 patients with macular diseases including macular hole, macular edema, central serous chorioretinopathy, age-related macular degeneration, choroidal neovascularization, epiretinal membrane, retinal pigment epithelium detachment, and retinitis pigmentosa. The MIT clinic developed a UHR ophthalmic OCT system that achieves 3-µm axial image resolution for imaging and performed comparative studies with UHR OCT and standard 10-µm-resolution OCT. Standard scanning protocols of six radial 6-mm scans through the fovea were obtained with both systems.
UHR OCT and standard-resolution OCT images were correlated with dilated ophthalmoscopy, fluorescein angiography, and indocyanine green angiograms to assess morphological information contained in the images. Correlations of UHR OCT images, standard-resolution OCT images, fundus examination, and/ or FA were demonstrated in full-thickness macular hole, central serous chorioretinopathy, macular edema, AMD, RPE detachment, epiretinal membrane, vitreal macular traction, and RP. UHR OCT and standard OCT exhibited comparable performance in differentiating thicker retinal layers, such as the retinal nerve fiber, inner and outer plexiform, and inner and outer nuclear. UHR OCT was better at differentiating finer structures or structures with lower contrast, such as the ganglion cell layer and external limiting membrane. UHR OCT confirmed the interpretation of features, such as the boundary between the photoreceptor inner and outer segments, which is also visible in standard OCT. UHR OCT's improved resolution was especially advantageous in assessing photoreceptor morphology.
(Ophthalmology 2005;112:1922-35.)
Ko TH, Fujimoto JG, Schuman JS, Paunescu LA, Kowalevicz AM, Hartl I, Drexler W, Wollstein G, Ishikawa H, Duker JS.
A Drug for Surgery-Related Night Vision Woes
Diluted aceclidine was found to be to be an effective and safe treatment for night vision disturbance following refractive surgery in a study at the Department of Ophthalmology in the Istituto Clinico Humanitas, Rozzano-Milano, Italy.
The double-masked, randomized clinical trial included 30 patients (60 eyes) with chronic night vision disturbance after refractive surgery. Patients were randomly allocated to receive placebo, aceclidine 0.016%, or aceclidine 0.032%. Drugs were administered once or twice daily. Anterior segment, haze, uncorrected visual acuity, best corrected visual acuity, intraocular pressure, corneal maps and scotopic pupil size were determined at baseline and at follow-up examinations (15 and 30 days after inclusion). Halos and double vision four-step scales were built to determine subjective grading of night vision disturbance, and the root mean square (RMS) was calculated to determine objective changes in night-vision disturbance.
The effect of diluted aceclidine started about 15 minutes after instillation and lasted for about five hours. No difference between the two dilutions was found. Thirty-nine of 40 treated eyes showed a reduction in night-vision disturbance. The mean reduction in halos and double vision grading was 1.42 ±0.5 (SD) and 1.14 ±0.4, respectively. A mean decrease in pupil size of 2.5 mm was measured. Thirty minutes after the instillation of diluted aceclidine, the topography-derived wavefront error showed a statistically significant reduction in RMS values (total, spherical, astigmatic, coma and higher order), which was maintained for five hours. Transitory conjunctival hyperemia was the only side effect reported.
(J Cataract Refract Surg 2005;31: 1764-1772)
Randazzo A, Nizzola F, Rossetti L, Orzalesi N, Vinciguerra P.
VISION Study Group Reports
Early detection and treatment with pegaptanib (Macugen, Eyetech Pharmaceuticals) may result in superior vision outcomes in patients with neovascular AMD, says the VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group. Their most recent report assessed the vision benefit of treating early subfoveal CNV secondary to AMD.
The exploratory analysis looked at the week 54 vision outcomes of subject subgroups with early disease who received 0.3 mg of pegaptanib (Groups 1 [n=34] and 2 [n=30]) or sham injections (usual care). Pegaptanib responder rates (loss of <15 letters of visual acuity) were 76 percent in Group 1 and 80 percent in Group 2 vs. 50 percent in Group 1 and 57 percent in Group 2 for usual care (p=0.03 and p=0.05 respectively). Compared with subjects assigned to pegaptanib, those receiving usual care in Group 1 lost on average 11.1 more letters of visual acuity and 12.7 more letters in Group 2 (p< 0.01 and p< 0.006 respectively). Subjects assigned to usual care were about 10 times more likely to have severe vision loss than were those treated with pegaptanib (Group 1, 29 percent vs. 3 percent, respectively; p< 0.01). In Group 1, 12 percent of pegaptanib-treated subjects gained ≥15 letters of VA vs. 4 percent receiving usual care; 20 percent of Group 2 pegaptanib-treated subjects gained ≥15 letters of visual acuity versus none of the usual care subjects.
(Retina 2005;25:815-827)
The VISION Clinical Trial Group.




