Many physicians and scientists have dreamt of the day when technology would be able to restore sight to the blind. Though it's still not possible to grant perfectly normal vision to those without it, researchers continue to redefine the boundaries of what's possible using solid-state technology. In this article, Review looks at the current state of one of the first forays into artificial vision, the Artificial Retina Project.
The Artificial Retina Project is a joint-venture whose sponsors include the U.S. Department of Energy, USC's Doheny Eye Institute and Second Sight Medical Products Inc. The concepts and technology used by the project have been under study since the mid- 1990s, but have only recently passed important safety checkpoints that will allow the researchers, headed by USC's Mark Humayun, MD, PhD, to hone the artificial retina's efficacy, as well.
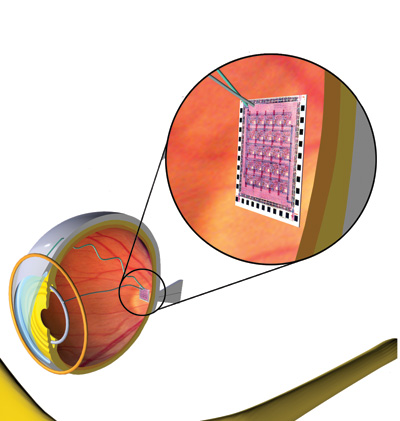 |
| The retinal implant uses 16 electrodes to stimulate the macula into seeing spots of light. Mark Humayun, MD |
The only human testing so far with the Artificial Retina has been with a 16-electrode array called the Model-1 device. It consists of 16 electrodes affixed to the retina via a tack. Patients wear glasses with a tiny camera embedded in the lens that captures images and sends them to a microprocessor, concealed in the side of the glasses, that converts the data to an electronic signal. An antenna in the lens wirelessly transmits the signal to a receiving antenna in the eye. The signal then travels to the retinal implant where it causes the implant to stimulate the remaining retinal cells, which send the image along the optic nerve to the brain. The inner components are powered by the outer through inductive power that doesn't require direct contact.
"We learned a lot from the 16-channel device study," says Dr. Humayun. "Specifically, a lot of what we learned was related to safety. Follow-up of six patients with the device over multiple years has shown that at least the device can be placed on the retina, and that when we test the patients' vision, the amount of electrical current required to elicit visual perceptions doesn't drift up or down. That was important because no one could ever say for sure whether after you put an implant in, years later it would require more current."
Though the Model-1 device was aimed primarily at testing safety and viability within the eye, the researchers were pleasantly surprised to find that it enabled patients to see light spots when certain electrodes were stimulated, and allowed them to differentiate between such objects as a paper cup or a knife in a high-contrast environment. "Not that they were seeing them, but the shapes could be differentiated from the background," notes Dr. Humayun. "One patient said a knife looks like a runway of lights, while a plate took on a saucer-like shape. This was impressive to us, because if you degraded your computer screen to 16 pixels, you couldn't see anything, but with these patients the brain was able to fill in an amazing amount of information."
The next device Dr. Humayun says he and his staff will be working on will be the "marketable" device, aimed not only at safety, but increased efficacy as well.
"It's got 60 channels, so roughly four times as many electrodes as the Model-1 device, and is about a quarter of the size," explains Dr. Humayun. "All of it fits around the eye, whereas in the Model 1, the electronics were behind the ear and the cable had to be tunneled along the temporal aspects of the head and then go into the eye. So it will be much easier to implant surgically in a much shorter procedure, about an hour and a half to get the implantation done."
To assess efficacy of the Model-2 device, they will perform visual acuity testing and testing of the activities of daily living to find out what these patients can really do with these devices. For example, they'll be trying to answer questions such as whether or not they prevent patients from having falls.
Two of the potential design issues with the larger 60-channel array are interference between electrodes, and tuning the electrodes. Interference could occur between channels, causing the brain to perceive two electrodes as one. This hasn't happened in the Model-1 device.
Tuning could be an issue because of the larger number of electrodes, each of which needs to be tuned for the particular patient's nervous system, like tuning a radio just right to receive a particular station. Each channel could take up to 30 minutes to tune, meaning a 60-channel device could take a huge amount of time to tune properly. "We have some interesting thoughts though, on how to fit the array much quicker," says Dr. Humayun. "Basically what you learn from a few electrodes you can apply to the rest. But you can't solve these challenges until you're in a patient."
The design of the new model and the pre-clinical testing data are being reviewed by the Food and Drug Administration, and Dr. Humayun hopes to be able to begin implanting the device sometime in 2006.
"Things are very exciting, because the first device far outperformed what we thought it would do," says Dr. Humayun. "The amount of information the brain could fill in was amazing. The encouraging results we got with this low resolution device have really made us optimistic about the 60-channel device."



