Various studies have suggested that the incidence rate of keratoconus is between 0.2 and 4,760 per 100,000 people and 1.5 and 25 per 100,000 people/year.1 Previous treatment options for the masses included cornea transplant, penetrating keratoplasty, Intacs and various types of lenses-rigid gas permeable lenses, scleral lenses and soft contact lenses for milder cases. But what if every keratoconus patient had a host of novel treatment options available to them?
Corneal collagen cross-linking has been the cutting-edge treatment option for keratoconus,2 and a number of ophthalmic medical technology and pharmaceutical companies have been pioneering the latest advancements in corneal cross-linking, and, in some cases, researchers have been developing management techniques in tandem with this technology. To appreciate the scope of the developments in keratoconus management, here’s a list of devices and techniques that may eventually come to your practice.
CXLens (TECLens)
 |
| TECLens’ partnership with SERVImed will allow for the incorporation of Ribocross with CXLens treatment. The riboflavin solution is maintained in a scleral lens reservoir. (Courtesy TECLens) |
TECLens announced its pilot trial results for its transepithelial corneal crosslinking contact lens device, CXLens, in 2021. Now, the company is gearing up for Phase II/III of U.S. Food and Drug Administration trials.
CXLens is an on-eye UV-A light-emitting contact lens device that operates using fiber optics for corneal cross-linking. Its main use is for the treatment of keratoconus, but Roy S. Chuck, MD, the Chairman of Ophthalmology at Albert Einstein College of Medicine and cofounder of TECLens, notes that the device is beginning to be applied for treating refractive errors.
“The original pilot trial publication of the CXLens described that the device was putting the UV light source on a controlled contact lens,” Dr. Chuck explains. “Since then, we’ve added a feedback element. This is an ultrasound biosensor in the lens. So, in real time, you can cross-link, and you can go ahead and monitor the cross-link and really monitor the cornea as it stiffens.” The idea for this additional technology was to provide surgeons with ample control while performing the cross-linking procedure. To further strengthen the control of the procedure, TECLens has developed more advanced features.
“We’re also starting to develop more computer modeling to go ahead and use in conjunction with the cross-linking and ultrasound biosensors,” Dr. Chuck continues. “The ultimate goal is control. If you can control shape change and stiffening change in the cornea using these three elements of cross-linking, feedback of ultrasound and computer modeling to help guide the whole thing, you should have something that’s quite precise and hopefully can control the cornea and it’s reshaping in all kinds of ways.”
In addition to these novel approaches to development, TECLens has partnered with SERVImed Industrial Spa to combine their product Ribocross (riboflavin 0.1%; dextran 10%; Vitamin E TPGS solution) with CXLens technology. Although Ribocross has received Orphan Drug Status from the FDA, Dr. Chuck explains that this solution won’t be introduced with CXLens until the device is fully developed and approved.
 |
| The CXLens platform includes a compact system with lenses that emit UV-A light rays for cross-linking treatment. Two lenses are attached to the device to perform cross-linking on both eyes simultaneously. (Courtesy TECLens) |
According to the pilot study,3 nine corneal transplant candidates with advanced keratoconus received a scleral lens reservoir. The lenses were fixed with 0.007% benzalkonium chloride preserved with 0.25% riboflavin-monophosphate. After 30 minutes, the lens was removed, and subjects received treatment from the CXLens (375-nm UV-A light at 4mW/cm2). After another 30 minutes, each subject had received a dose of 7.2 J/cm2. During the follow-up after six months, subjects who were treated with the CXLens had an average -1.0 ±1.6 D decrease in maximum keratometry.
“Our goal has always been to address the issue of precision medicine, patient specific medicine and customized control,” says Dr. Chuck. “As we go forward, it’s all about treating the patient using their own data rather than trying to guess, and we’re trying to take the guesswork out of what we do by doing everything with ultrasound monitoring and finite element modeling. Hopefully, this will give us the most precise correction possible.”
Glaukos
Glaukos was the first company to have an FDA-approved corneal cross-linking device. Their latest FDA approved epithelium-off riboflavin solution, Photrexa, is used along with Glaukos’ iLink Corneal Cross-Linking system. Currently, Glaukos is developing their epithelium-on platform, Epioxa, and a third-generation corneal cross-linking system.
• Epioxa. In 2023, Glaukos announced that the Phase III FDA trial for Epoxia had reached enrollment completion. This confirmatory pivotal trial has enrolled 312 eyes for epi-on corneal cross-linking treatment. The subjects will be randomly selected in a 2:1 ratio to receive Epioxa therapy or a placebo. This trial will evaluate whether the therapy is safe and effective when attempting to stop the progression of keratoconus and/or reduce the maximum corneal curvature (Kmax) for keratoconic eyes. In a press release, Glaukos stated that the primary endpoint that needed to be achieved was the mean change in Kmax from baseline through a 12-month follow-up period. If there’s a significant difference between the treated subjects and the control’s primary endpoint results and if there’s a ≥1 D difference, then the study would be considered a success by the FDA.
• NXL UV-A Device (Third Generation). Additionally, Glaukos reported its findings on the safety of its third-generation corneal cross-linking system with various riboflavin doses.4 The company reported that three subjects received a high dose of UV-A while nine subjects received low, medium or high doses of UV-A in a dose-escalating manner. Every patient received epi-on therapy for this study. In 2023, only a total of 17 percent of subjects completed the six-month follow-up period and showed mild adverse events similar to those observed in conventional cross-linking treatments. At ASCRS 2024, Glaukos reported the 12-month data from the study, and they noted no change in adverse events from the six-month follow-up.
• IVMED-80. After Glaukos entered a licensing agreement with iVeena to develop and commercialize IVMED-80 (copper sulfate eye drops), the pharmacological treatment is currently undergoing Phase III FDA trials. In 2020, under the leadership of iVeena, a team of researchers evaluated the safety and preliminary efficacy of the eye drops in Phase I/IIa FDA trials.5
IVMED-80 was developed to increase lysyl oxidase, a cross-linking enzyme that, when decreased, is linked to keratoconus.6 During the 26-week trial, a total of 36 patients were randomly divided equally into three treatment groups: Group 1 received IVMED-80 treatment for six weeks, then stopped treatment for the remaining 20 weeks, Group 2 received IVMED-80 treatment for 16 weeks, then stopped treatment for the remaining 10 weeks, and Group 3 received placebo eye drops for 16 weeks, then stopped treatment for the remaining 10 weeks.
According to the study, no adverse events were reported during the 16 weeks of treatment. At 16 weeks, 19 IVMED-80 patients showed a 1-D reduction of Kmax, while the placebo group progressed by 0.46 D of Kmax. Subjects in Group 1 showed a 0.46 D reduction of Kmax at two months, but their eyes reverted back to baseline by 16 weeks. The researchers reported that there was no clinically significant effect on IOP or other ocular findings.
EpiSmart (Epion Therapeutics)
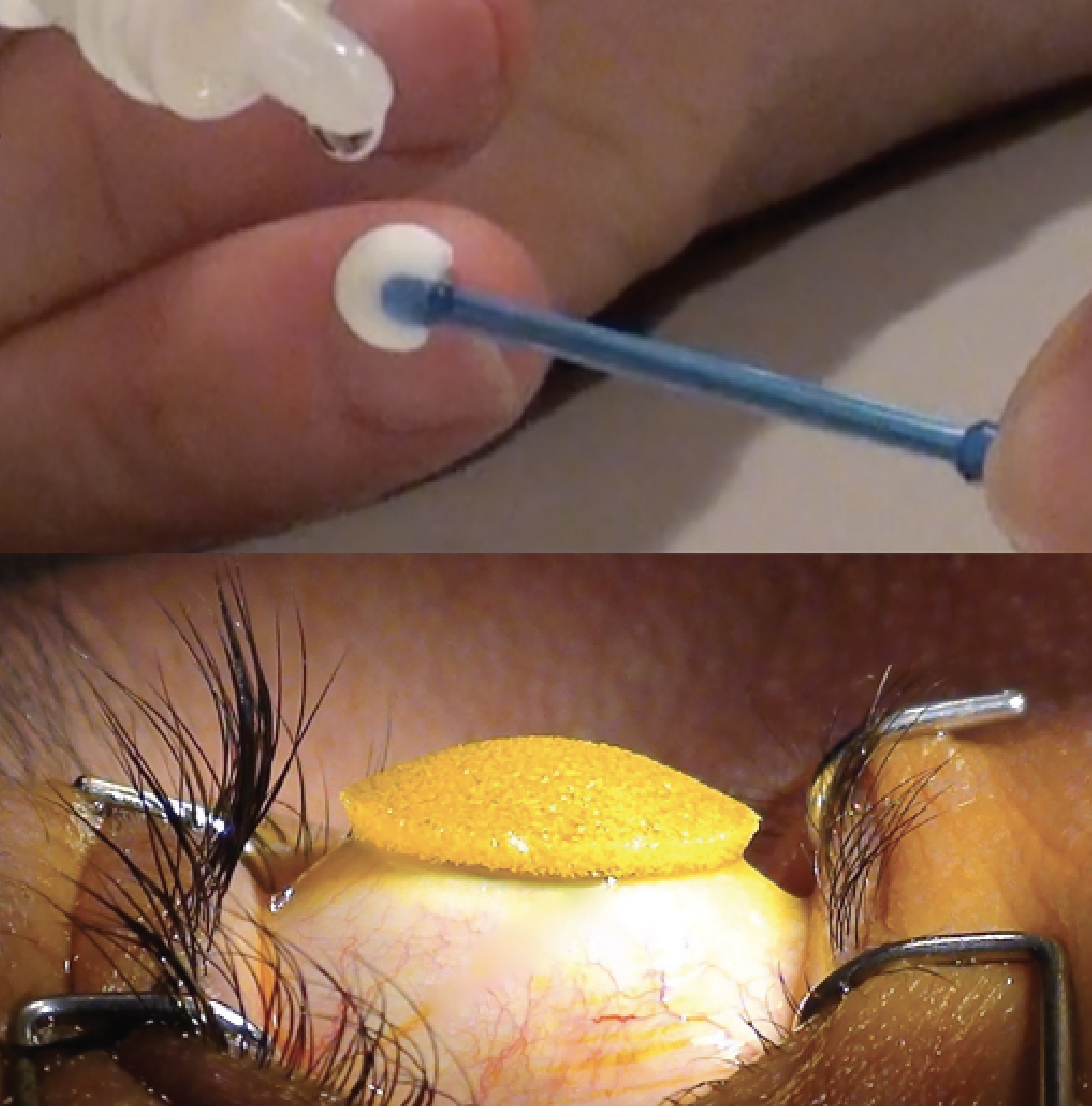 |
| EpiSmart’s EpiPrep disposable wand (Top) and disposable loading sponge (Bottom). (Courtesy EpiSmart) |
According to their website, Phase III FDA trials (Apricity A and Apricity B) for Epion’s minimally invasive keratoconus treatment, EpiSmart, commenced in 2023. They plan on enrolling 800 subjects from across the United States, including patients as young as 8 years old, as long as they’ve met the enrollment criteria.
This epi-on platform includes RiboStat, a high concentrated riboflavin/sodium iodide solution. This is administered using EpiPrep, a two-part system including a disposable wand to enhance epithelial permeability and a disposable loading sponge to sustain high drug concentration during stromal loading. This is when the EpiSmart UV-A device is introduced to perform epi-on corneal cross-linking.
During Phase II FDA trials,7 Epion researchers enrolled 2,228 subjects who were assessed at six and 12 months postoperatively. The subjects were randomly divided into three different treatment groups, each receiving various doses of UV-A treatment. The doses administered were 2.4 J/cm2 over 20 minutes, 3.6 J/cm2 over 20 minutes and 3.6 J/cm2 over 30 minutes. The primary endpoint observed by the researchers was logMAR CDVA, while secondary endpoints included logMAR UCVA, Kmax and minimum corneal thickness.
Researchers reported that 1,922 subjects were diagnosed with keratoconus and the other subjects had postsurgical or other ectasias. The primary endpoint was achieved and resulted in a significant improvement in CDVA for all groups. UCVA and Kmax secondary endpoints were significant as well, but minimum corneal thickness remained unchanged. Although no serious adverse events were reported, 195 cases (8.7 percent) had at least one adverse event. The most prevalent complication was a mild corneal epithelial defect, which was found in 31 cases (1.4 percent).
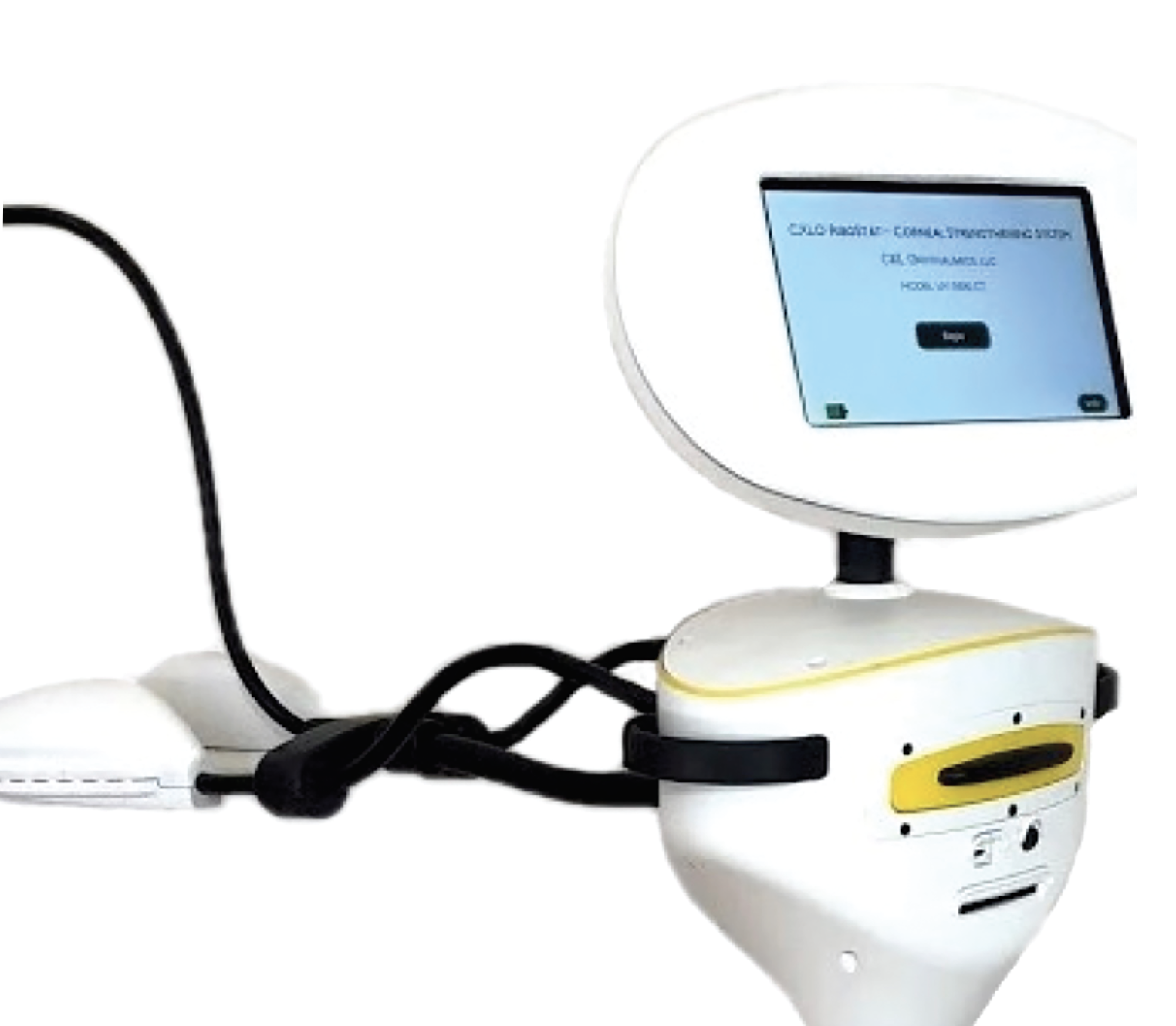 |
| EpiSmart’s corneal collagen cross-linking system uses an epi-on approach. (Courtesy EpiSmart) |
Omni CXL (Micro Medical Devices)
According to Micro Medical Devices, the Omni CXL is a transportable corneal cross-linking system awaiting market approval in the U.S. This device received it’s CE mark in 2006 for European distribution and now it’s in clinical use in over 100 countries.
 |
| Omni CXL portable system can be stored away and transported with the compatible case. This is useful for traveling or medical mission trips the company says. (Courtesy Micro Medical Devices) |
Micro Medical Devices explains that the device can be tuned to output energy from 3 mW to 45 mW per cm2 with automatic time adjustment. The system is built with an integrated infrared camera for eye tracking to ensure proper distance. Both the tracking area and the threshold can be individually set. Automatic riboflavin selection recommendation is available when using the integrated pachymeter. Additionally, patient data and treatment settings can be exported as a PDF and saved to the patient’s medical record.
Micro Medical Devices filed for FDA market approval in 2018.
CAIRS
“Corneal allogenic intrastromal ring segments, or the CAIRS technique, was a method that started in 2015 to treat keratoconus,” says Soosan Jacob, MD, the director and chief of Dr. Agarwal’s Refractive and Cornea Foundation and developer of CAIRS. “CAIRS represents the use of any kind of allogenic tissue which is inserted into the cornea as segments, which could be either uniform segments or customized segments.
“Since about 2018, we started customizing CAIRS,” continues Dr. Jacob. “This is true customization, not just based on the arc length or location, but actually based on the individual patient’s keratometric maps, thickness maps and other maps. What you have to realize is that no two keratoconic patients are similar. Even if they fall into broadly the same phenotypic classes. Because we customize for every zone of the patient’s map, we have variable thicknesses available with CAIRS, which gives you much better customization and therefore improved visual acuity results.
“We use a double-bladed design, which is patented, for cutting the CAIRS segments, and this gives us the ability to really exquisitely customize to each patient, or rather to each patient’s type of keratoconus,” says Dr. Jacob. “CAIRS is a minimally invasive surgery because the entire surgery is done through about a 1-mm incision or 1.5-mm incision and the channels are created using a femtosecond laser. So, we use the laser to create the CAIRS channel and then implant the CAIRS segments into the channels.”
When it was first introduced, surgeons would make a manual incision and implant the donor tissue with SK Intacs as a guidewire.8 Now, many studies are observing the safety and efficacy of femtosecond-assisted CAIRS procedures. At ASCRS 2024, Bianca N. Susanna, MD, reported on the latest research findings for femtosecond laser assisted personalized CAIRS implantation in keratoconic eyes.9 After three months, subjects’ UDVA improved from 0.01 ±0.06 preoperatively to 0.29 ±0.2 on the last follow-up day. Also, CDVA improved from 0.29 ±0.13 to 0.5 ±0.24. A total of 21 patients gained two or more Snellen lines of CDVA. Furthermore, spherical equivalent (-8.87 ±2.07 D to -2.90 ±4.45 D) and cylinder (-3.75 ±2.11 D to -2.35 ±0.91 D) improved for subjects. It was reported that topographic astigmatism increased from 4.20 ±2.75 to 5.03 ±2.46. During the follow-up period, no serious adverse events were observed.
After the allogenic tissues are implanted, the cornea undergoes cross-linking treatment. Dr. Jacob explains that both conventional accelerated cross-linking and accelerated contact lens-assisted cross-linking can be employed during the procedure, but each method is used for specific cases.
“When the minimum stromal thickness is less than 400 µm, we go in with CACXL, which is contact lens-assisted cross-linking,” explains Dr. Jacob. “If there’s more than 400 µm, we do conventional accelerated cross-linking. In both these situations, the power and the duration that we use is 10 mW/cm2 for nine minutes.”
Published in 2014, the first study on contact lens-assisted cross-linking showed the safety and effectiveness of the treatment, proponents say.10 This treatment uses an ultraviolet barrier-free soft contact lens that’s placed on the patient’s cornea after being soaked in iso-osmolar riboflavin 0.1% for 30 minutes. As Dr. Jacob stated previously, the necessary corneal thickness for CACXL is less than 400 µm, and this study shows that the minimum thickness requirement for CACXL treatment is 350 µm. This method thickens the cornea to more than 400 µm, which is why it’s applied when a patient is seen with a stromal thickness of less than 400 µm.
Dr. Jacob adds that CACXL has its advantages when combined with CAIRS. She explains that in some cases, the thickness of the cornea is less than the minimum thickness needed for CACXL. After implanting the allogenic tissue into the eye, the cornea thickens, allowing patients to be treated with CACXL.
“We have done this kind of approach in some cases of topographic-guided PRK where the patient progressed and topographic-guided PRK was done with CXL,” Dr. Jacob mentions. “TGPRK with CXL was done for patients with keratoconus and after a few years a patient’s keratoconus progressed and they came to us. The patient had extreme thinning and fortunately it was in the same area where they had CAIRS implanted. Now, since it was so thin, we wouldn’t have been able to do a CACXL or CXL directly without CAIRS, but because we already placed CAIRS within this patient, we were able to increase the thickness of the cornea immediately in the thin zone because that was where the CAIRS was implanted, and it could be followed up with cross-linking.”
Now, common transplantation adverse events may occur, but Dr. Jacob doesn’t view this as an issue. “CAIRS can be associated with complications, but you have to remember that this is a reversible and adjustable procedure. If for some reason the patient doesn’t like the visual quality, then you can just reverse it by removing the CAIRS. On the other hand, if you feel that you need to get more or less results, you can also adjust the CAIRS segment easily.”
There are many devices and techniques in the pipeline for keratoconus management, but there’s more work that needs to be done. Each device mentioned in this article is still undergoing FDA trials and the CAIRS technique is advancing as more researchers introduce novel ways to create incisions, customize ring segments and perform different corneal cross-linking methods. It seems like minimally invasive treatment options for keratoconus are going to continue to grow, and it’ll be exciting to see what’s next on the horizon.
Dr. Chuck is the cofounder of TECLens. Dr. Jacob has no financial interests to disclose.
1. Santodomingo-Rubido J, Carracedo G, Suzaki A, et al. Keratoconus: An updated review. Contact Lens and Anterior Eye 2022;45:3.
2. Feldman BH, Halfpenny C, Bernfeld E, et al. Corneal collagen cross-linking. https://eyewiki.aao.org/Corneal_Collagen_Cross-Linking. Accessed on March 28, 2024.
3. Dackowski EK, Logroño JB, Rivera C, et al. Tranepithelial corneal crosslinking using a novel ultraviolet light-emitting contact lens device: A pilot study. Translational Vision Science & Technology 2021;10:5.
4. Smith V, Heersink SB, Hersh P, et al. Riboflavin ophthalmic solution activated in situ with a novel scanning laser-based bioactivation system, the NXL UV-A device, demonstrates safety of corneal cross-linking for the treatment of keratoconus. Investigative Ophthalmology & Visual Science 2023;64:8:1675.
5. Molokhia S, Muddana SK, Hauritz H, et al. IVMED 80 eye drops for treatment of keratconus in patients -Phase 1/2a. Invest Ophthalmol Vis Sci 2020;60:2587.
6. Dudakova L, Jirsova K. The impairment of lysyl oxidase in keratoconus and in keratoconus-associated disorders. J Neural Transm (Vienna) 2013;120:6:977-82.
7. Epstein RJ, Belin MW, Gravemann D, et al. EpiSmart crosslinking for keratoconus: A Phase 2 study. Cornea 2023;42:7:858-866.
8. Susanna BN. Exclusively femtosecond-assisted personalized Cairs: Description of a novel technique for creation and insertion. https://ascrs.confex.com/ascrs/24am/meetingapp.cgi/Paper/102884. Accessed on April 1, 2024.
9. Susanna BN. Femtosecond laser assisted personalized Cairs implantation in keratoconic eyes. https://ascrs.confex.com/ascrs/24am/meetingapp.cgi/Paper/102832. Accessed on April 1, 2024.
10. Jacob S, Kumar DA, Agarwal A, et al. Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J Refract Surg 2014;30:6:366-72.