Presentation
A 72 year-old Caucasian male was referred to the Wills Eye Institute Glaucoma Service for consultation regarding treatment options for glaucoma in both eyes. Despite compliance with a topical medical regimen, the intraocular pressure in the left eye was persistently elevated.
Medical History
The past medical history consisted of hypercholesterolemia, hypothyroidism, prostate cancer and cutaneous melanoma. In 1984, pigmentary glaucoma OU was found and treated with argon laser trabeculoplasty to both eyes. This maintained satisfactory IOPs for many years off all topical glaucoma medications. Eventually, topical medications were reinstituted to control IOP in both eyes and in 1994, trabeculectomy was necessary for pressure control in the right eye. At the time of consultation at the Wills Eye Institute Glaucoma Service, the topical regimen included bimatoprost at bedtime OU and timolol once a day OS. In the past, an alpha-agonist was used but was discontinued after the patient developed severe eyelid edema.
Examination
On examination, best-corrected visual acuity was 20/20 OU with no afferent pupillary defect. By Goldman tonometry, IOP was 12 mmHg OD and 22 mmHg OS. Central corneal thickness was 579 µm OD and 603 µm OS. On gonioscopy, the anterior chamber angle was open OU and classified as D45r OU. The scleral spur was clearly visualized bilaterally and the surgical peripheral iridotomy OD was patent. Humphrey visual field demonstrated a cecocentral scotoma OD that had been stable over an 18-month period. During the same interval, a nasal step defect OS had been documented to progress.
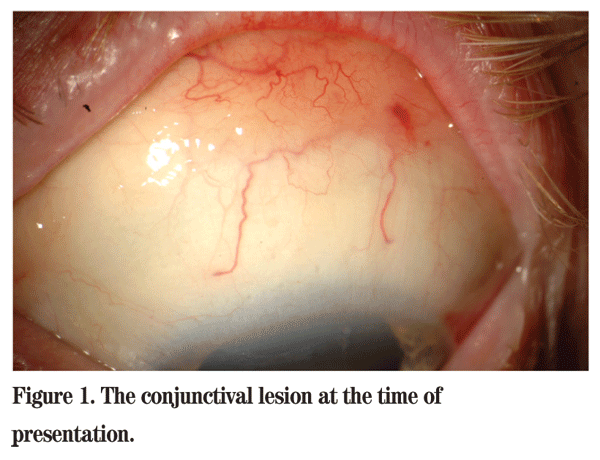
The optic discs OU demonstrated substantial temporal rim thinning but with healthy nasal rim. The estimated cup-to-disc ratio was 0.8 OU. Slit lamp examination revealed a superior bleb and mild nuclear sclerosis of the lens OD. The left eye displayed a salmon-colored subconjunctival mass occupying the entire superior fornix.
Diagnosis, Workup and Treatment
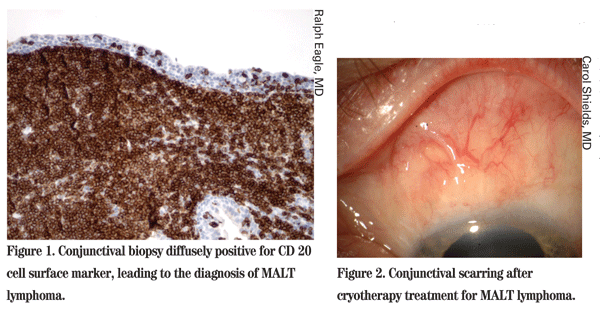
Using an unroofing technique, the conjunctival epithelium was horizontally incised on the posterior aspect of the mass and carefully elevated to expose the underlying subconjunctival lesion for complete excision. Cryotherapy was applied to adjacent tissues. The preserved epithelium was sutured closed. Histopathology revealed mucosal associated lymphoid tissue (MALT) lymphoma with strong positivity for CD20 cell surface marker (See Figure 1). Systemic evaluation for lymphoma was negative. After four months, the conjunctiva was well healed and glaucoma management was readdressed.
Visual acuity re-mained stable. In- traocular pressure by Goldmann tonometry was 13 mmHg OD and 26 mmHg OS. The left superior conjunctiva displayed linear conjunctival fibrosis extending to within 10 mm of the limbus and with substantial Tenon's fascia fibrosis and traction (See Figure 2). With elevated IOP on maximal medical therapy and a compromised optic nerve OS, it was believed that surgical intervention was necessary for better IOP control.
There was consideration for several procedures, given the extensive conjunctival scarring. A superior trabeculectomy would be difficult with the marked conjunctival fibrosis superiorly. A tube shunt placement inferiorly was an option. In the end, it was concluded that a canaloplasty would offer the most advantageous initial surgical approach, as this procedure could be performed despite the limbal scarring. Canaloplasty was performed OS without complication. At three month follow-up, IOP was 16 mmHg OS and the patients was off all topical glaucoma medications.
Discussion
With advancements in technology, the armamentarium available for glaucoma surgery has become more extensive over the past several years. Although many still view trabeculectomy as the gold standard for surgical glaucoma treatment, there are situations in which this procedure is not a practical option, especially when there is extensive conjunctival scarring, thinning or adhesion. Non-penetrating glaucoma procedures, such as canaloplasty, provide an alternative when conventional methods are not feasible.
Non-penetrating surgical glaucoma procedures are unique in that the anterior chamber is not violated. This strategy was first attempted in the 1960s with sinusotomy, a procedure on which one-third to one-half of Schlemm's canal was unroofed to restore the trabeculo-canalicular outflow path.1 The limitations of operating microscopes at the time made these procedures difficult to perform and yielded unpredictable results. In the 1990s, there was a resurgence of interest in non-penetrating surgical glaucoma procedures with deep sclerectomy and viscocanalostomy. However, comparison studies have shown that trabeculectomy demonstrates better IOP-lowering results.2 In these procedures, only a section of Schlemm's canal could be accessed. Later development of a 250-µm, flexible microcatheter by iScience allowed access to the entire length of Schlemm's canal and this lead to the advent of canaloplasty.
In canaloplasty, identifying Schlemm's canal is crucial. In order to gain access to it, a superficial partial-thickness, limbus-based scleral flap is initially made. Within the parameters of this flap, a second, deeper partial-thickness scleral flap is constructed. The dissection of the deep flap is then carried forward until Schlemm's canal is unroofed. The microcatheter, which has a lighted tip, is threaded into and around the circumference of the canal while viscoelastic is being injected. Once the lighted tip of the microcatheter has completed 360 degrees and exited, a 10-0 polypropylene suture is tied to this end.
The microcatheter is then pulled around in the reverse direction so that the suture traverses the entire length of Schlemm's canal. The suture loop is then secured with locking knots so that the canal and trabecular meshwork tent inwards. This technique functions according to the hypothesis that the permeability of the trabecular meshwork is improved with the tented opening of the channels. The deep scleral flap is amputated, and the superficial flap is secured with watertight sutures.3
An ongoing prospective study is looking at 127 patients in
Since the anterior chamber is not violated in canaloplasty, there is no bleb and there is hope to avoid many of the complications seen with trabeculectomy (i.e. blebitis, endophthalmitis, late-onset bleb leaks, hypotony, choroidal effusions, cataract formation, and bleb encapsulations). The aforementioned study also showed that the most common adverse events associated with canaloplasty were hyphema, early elevated IOP, bleb formation, late elevated IOP, wound hemorrhage, Descemet membrane detachment, suture extrusion through the trabecular meshwork and hypotony.4
With only two years of data, more research is necessary to determine the short-term and long-term efficacy of canaloplasty when compared with standard technique of trabeculectomy. However, it is clear that this procedure offers glaucoma surgeons a creative option in the operating room when facing a glaucoma patient with conjunctival scarring or anatomic restrictions.
The author would like to thank L. Jay Katz, MD, Wills Eye Institute Glaucoma Service, Carol Shields, MD, of the Wills Eye Institute Department of Ocular Oncology, and Ralph Eagle, MD, of the Wills Eye Institute Department of Pathology, for their time and assistance.
1. Krasnov MM. Externalization of Schlemm's canal (sinusotomy) in glaucoma. Brit J Ophthal 1968;52:157-161.
2. Godfrey DG, Fellman RL,
3. Lewis RA, et al. Canaloplasty: Circumferential viscodilation and tensioning of Schlemm canal using a flexible mircocatheter for the treatment of open-angle glaucoma in adults: Interim clinical analysis. J Cataract Refract Surg 2007;33:1217-26.
4. Lewis RA, et al. Canaloplasty: Circumferential viscodilation and tensioning of Schlemm canal using a flexible mircocatheter for the treatment of open-angle glaucoma in adults: Two- year interim clinical study results. J Cataract Refract Surg 2009;35:814-24.