Diagnosis, Workup and Treatment
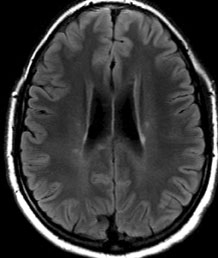
Given the patient’s motility deficits with convergence-retraction nystagmus, a lesion in the dorsal midbrain was suspected. The differential diagnosis included demyelinating disease (e.g., multiple sclerosis, acute disseminated encephalomyelitis), tumor, hydrocephalus, hemorrhage, infarction, aneurysm, inflammation and infection. The patient was admitted and underwent an MRI of the brain and brainstem. Initial interpretation of the MRI by the radiology resident revealed multiple punctate white matter lesions, but none in the dorsal midbrain to account for the patient’s symptoms (See Figure 2). Subsequent laboratory tests including a lumbar puncture showed 11 white blood cells in the cerebral spinal fluid, normal protein and glucose, no oligoclonal bands or abnormal immunoglobulin G (IgG) levels. Evaluation for Lyme disease, sarcoid and vasculitis was negative.
Review of the MRI with the attending neuroradiologist the following day revealed a small area of enhancement in the superior dorsal midbrain (See Figure 3). Faint periventricular areas of enhancement were seen hugging portions of the undersurface of the corpus callosum, which is typically suggestive of demyelinating disease.
The patient was initially placed on pulsed intravenous steroids. The patient tolerated treatment well with dramatic improvement of his vision and motility. He was discharged home on high dose IV steroids and transitioned to high-dose, high-frequency interferon (IFN) β-1a. He has been monitored with serial MR imaging without evidence of new lesions or clinical exacerbations.
 |
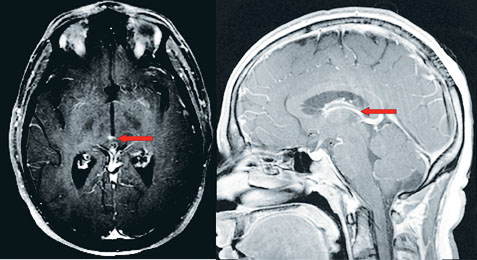 |
| Figure 2. MRI T2 Flair axial image demonstrating multiple periventricular gadolinium-enhancing lesions. | Figure 3. MRI T1 post-contrast sagittal image demonstrating small dorsal midbrain lesion (left). MRI T2 post-contrast axial image demonstrating a small area of enhancement in the superior dorsal midbrain (right). |
Discussion
The rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) and the rostral interstitial nucleus of Cajal (RIC) are located in the midbrain and serve as the brainstem center for vertical gaze. Diffuse areas of the cerebral cortex including the frontal eye fields as well as the vestibular nuclei and pretectal area send signals to the riMLF, which in turn send upgaze neurons to the RIC. The RIC serves as an integrator to sustain vertical gaze by sending neurons directly and indirectly to the oculomotor and trochlear nuclei via neurons that cross in the posterior commissure.
Dorsal midbrain syndrome, or Parinaud’s syndrome, is characterized by varying combinations of the following: paralysis of upgaze; light-near dissociation; convergence-retraction nystagmus; eyelid retraction or Collier’s sign; and skew deviation. It is important to note that convergence-retraction nystagmus describes abnormal convergence of saccades, rather than a true nystagmus, or abnormality of slow eye movements. The differential diagnosis of dorsal midbrain syndrome includes pineal cyst, pinealoma, multiple sclerosis, demyelinating disease, stroke, mass, toxoplasmosis, hydrocephalus, Niemann-Pick disease, Wilson’s disease, kernicterus and barbiturate overdose.
A clinically isolated syndrome (CIS) is defined by a set of neurologic symptoms lasting for at least 24 hours affecting the optic nerve, brainstem, cerebellum, or spinal cord that is suggestive of CNS demyelination or inflammation. Ninety percent of patients with MS present with CIS. Clinically definite MS (CDMS) is diagnosed when further clinical attacks or new MRI lesions occur. CIS consisting of unilateral optic neuritis, incomplete transverse myelitis, internuclear ophthalmoplegia, trigeminal neuralgia, and paroxysmal attacks are particularly suggestive of MS.
Early revisions to the McDonald Criteria for the diagnosis of MS required demonstration of dissemination in space (DIS) and dissemination in time (DIT), usually by MRI findings. The most recent revisions to the McDonald Criteria in 2010 define DIS as at least one T2 lesion in at least two of four locations considered characteristic for MS (e.g., juxtacortical, periventricular, infratentorial, spinal cord) with lesions within the symptomatic region excluded in patients with brainstem and spinal cord syndromes. DIT was defined as a new T2 and/or gadolinium-enhancing lesion(s) on follow-up MRI with reference to a baseline scan, irrespective of timing at baseline and simultaneous presence of asymptomatic gadolinium-enhancing and non-enhancing lesions at any time. According to the new revisions, presence of elevated IgG or oligoclonal bands do not reduce the MRI requirements.
Additional studies examined the significance of paraclinical tests in the diagnosis of CIS and the development of CDMS. Brain MRI and olicoglonal IgG band (OCGB) detection have been useful in predicting conversion to CDMS. Jaime Masjuan, MD, and colleagues showed that OCGB had a sensitivity of 91.4 percent and a specificity of 94.1 percent for early prediction of conversion to CDMS. MRI criteria had a sensitivity of 74.2 percent and a specificity of 88.2 percent.
The Barkhof MRI criteria consist of demonstration of three of four of the following: 1) presence of at least one gadolinium-enhancing lesion or nine in the T2-weighted images; 2) presence of at least three periventricular lesions; 3) presence of at least one juxtacortical lesion; 4) presence of at least one infratentorial lesion.
Treatment for CIS can delay the onset of a second attack, reduce clinical relapses, slow disease progression, and reduce new MRI lesions. Results from the Controlled High-Risk Subjects Avonex Multiple Sclerosis Prevention Study (CHAMPS) showed that patients who received once weekly intramuscular injections with 30 µg of IFNβ-1a were 15 percent less likely to have subsequent events than patients who received placebo over three years. The Early Treatment of MS (ETOMS) study examined once weekly subcutaneous injections with 22 µg of IFNβ-1b and showed that treated patients were 11 percent less likely to have subsequent clinical attacks than untreated patients in two years.
Similarly, the Betaseron in Newly Emerging MS for Initial Treatment (BENEFIT) randomized patients to receive 250 µg of IFNβ-1b every other day for two years. Treated patients were 18 percent less likely to develop a subsequent attack after two years. All patients who developed a second attack were treated with 250 µg of IFNβ-1b every other day for three years. The immediate-treatment group showed a 14 percent decrease in number of attacks than the delayed-treatment group.
The Presenting with a CIS (PRECISE) study treated patients with unifocal CIS and abnormal MRI with 20 mg/day of glatiramer acetate. The treatment group was 18 percent less likely to develop CDMS and showed a 61-percent decrease in new T2 lesions and a 60-percent decrease in new contrast lesions when compared to the placebo group.
While previous studies show the benefits of injectable medications, there are newer oral alternatives on the horizon. Fingolimod is the first oral disease-modifying drug approved by the Food and Drug Administration in 2011 for the treatment of multiple sclerosis. It is a sphingosine 1-phosphate receptor modulator that blocks the release of lymphocytes from lymph nodes preventing their recirculation into the CNS. Cladribine is an oral purine nucleoside analog that selectively targets lymphocytes and disrupts DNA synthesis promoting apoptosis. Cladribine’s efficacy in multiple sclerosis and drug safety are being studied. Other promising oral disease-modifying preparations include teriflunomide, laquinimod and BG 12 (dimethyl fumarate).
The author would like to thank Mark Moster, MD, of the Wills Eye Neuro-ophthalmology Service for his assistance with this case.
1. Brodal P. The Central Nervous System. Oxford: Oxford University Press, 2010.
2. Coyle PK. Early treatment of multiple sclerosis to prevent neurologic damage. Neurology 2008 Dec 9; 1(24 Suppl 3):S3-7.
3. Dalton CM, Brex PA, Miszkiel KA, et al. Application of the new McDonald criteria to patients with clinically isolated syndromes suggestive of multiple sclerosis. Ann Neurol 2002;52:47-53.
4. Frohman EM, Dewey RB, Frohman TC. An unusual variant of the dorsal midbrain syndrome in MS: Clinical characteristics and pathophysiologic mechanisms. Mult Scler 2004;10:322-5.
5. Masjuan J, Alvarez-Cermeño JC, García-Barragán N, et al. Clinically isolated syndromes: A new oligoclonal band test accurately predicts conversion to MS. Neurology. 2006;66:576-8.
6. Ontaneda D, Hyland M, Cohen JA. Multiple sclerosis: New insights in pathogenesis and novel therapeutics. Annu Rev Med 2012;63:5.1-16.
7. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011;69:292-302.
8. Tintore M, Rovira A, Rio J, et. Al. New diagnostic criteria for multiple sclerosis: Application in first demyelinating episode. Neurology 2003;60:27-30.



