As you know, our primary way of preventing glaucoma progression is by reducing intraocular pressure. Most procedures, including trabeculectomy, tube shunt implantation and most minimally invasive glaucoma surgeries (MIGS), reduce IOP by increasing the outflow of aqueous from the eye. The most notable exception has always been cyclophotocoagulation of the ciliary body (CPC), which alters this tissue and reduces its secretion of aqueous humor. (It may also increase outflow; more on that later.) For many years, this has been accomplished using either external lasers (transscleral cyclophotocoagulation, or TSCPC), or an endoscopic probe to target the tissue from inside the eye (endoscopic cyclophotocoagulation, or ECP). More recently, the use of a non-con-tinuous-wave laser pattern has created a new, less-tissue-altering way to accomplish this from outside the eye (micropulse cyclophotocoagulation, or MPCPC).
All three are effective at lowering IOP, and all three have benefits and risks associated with them. Here, I’ll share the pros and cons of each approach, along with pearls to help you make the most of whichever one you may be using.
Transscleral CPC
When performing transscleral cyclophotocoagulation—lasering from outside the eye—the three risks that we’re primarily concerned about are hypotony, phthisis and vision loss. These complications are rare, but they’ve been an issue since people started performing cyclodestructive procedures almost 100 years ago. That’s because, historically, ophthalmologists have waited until patients had end-stage disease to perform cyclophotocoagulation. End-stage patients—particularly those with neovascular glaucoma—tend to do poorly regardless of how you treat them.
 |
|
|
For example, one study of patients with advanced neovascular glaucoma compared the effectiveness of a cyclodestructive procedure and a Baerveldt tube shunt.1 Both groups had similar rates of complications; in both groups, vision decreased by almost 40 percent despite treatment, and both groups had an 8-percent risk of phthisis. So a tube shunt also produced less-than-ideal results in these patients.
 |
|
|
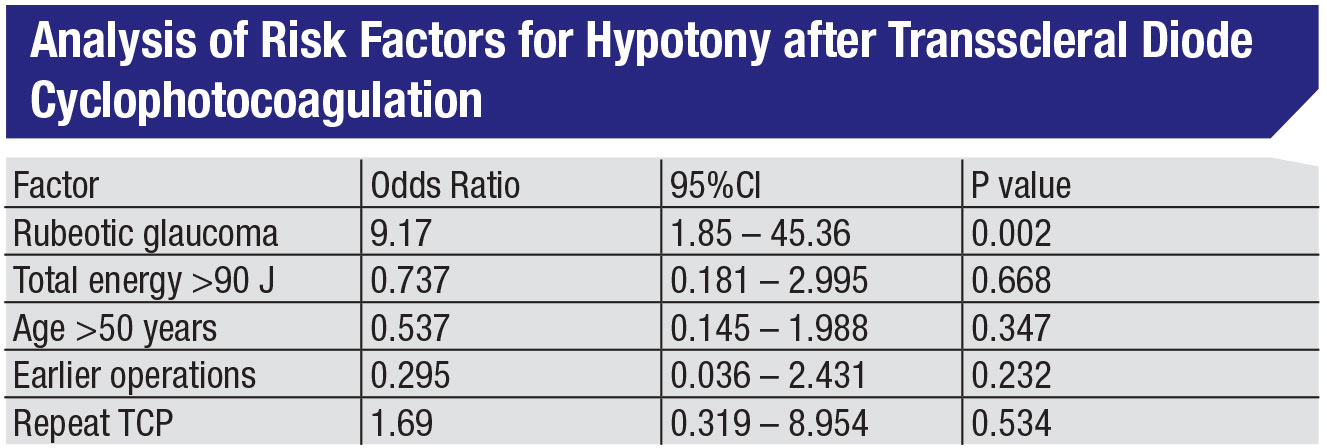
In fact, other studies have shown that neovascular glaucoma—i.e., advanced disease—is far and away the largest risk factor for developing hypotony following CPC.2 (See table, above.) As Harry Quigley, MD, noted in one of his studies involving TCP, “Although phthisis and enucleation occurred, they were uncommon and frequently associated with severe baseline status and complex additional ocular problems.”3
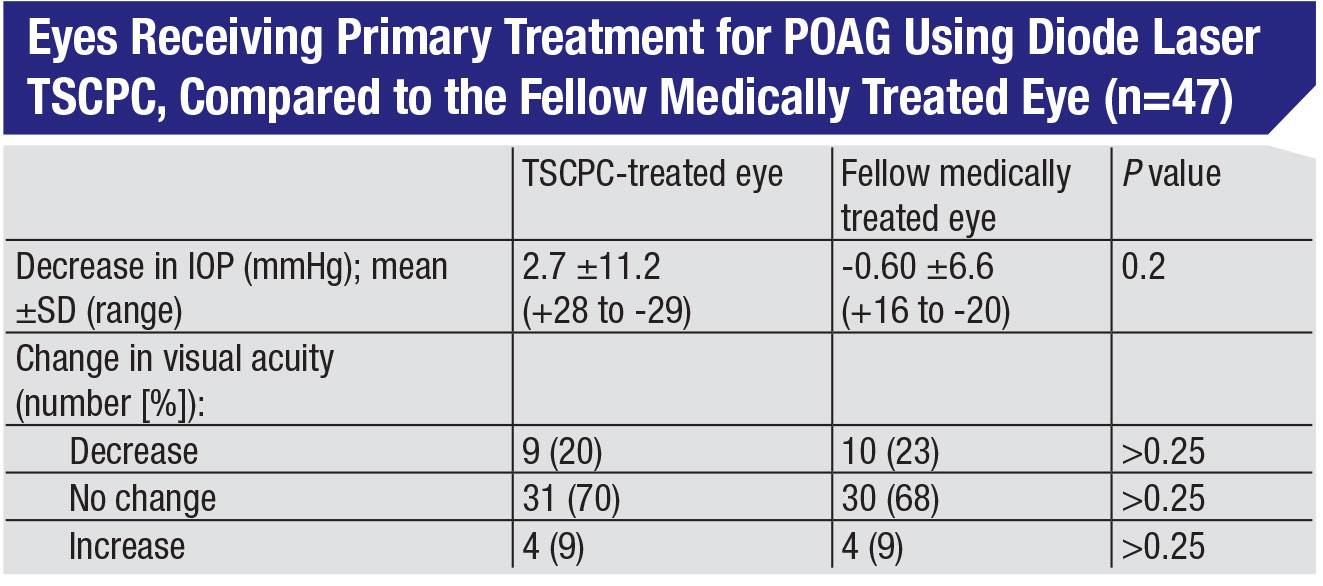
The fact that this procedure is safer than its reputation suggests has been confirmed by a number of studies. In studies in which TCP is used to treat glaucoma patients at an earlier stage of disease, not only is it effective, but patients tend to do quite well. For example, in one study, TPC was compared to traditional glaucoma drops as primary treatment in fellow eyes.4 Researchers found that in the 19 eyes that had baseline good vision, only one experienced a decrease in vision over the course of the study, and there was no significant difference in visual acuity between the fellow eyes at the final visit. (See table, p. 18.) Also, TCP can be effective as an adjunctive treatment in patients with glaucoma drainage implants, with a low rate of complications.5
The implication is clear: If you start with patients who have better prognoses, they’ll do better regardless of which therapy regimen you choose, and TSCPC is an option worth considering.
Endoscopic CPC
I think of endoscopic cyclophotocoagulation as the forgotten MIGS procedure. (I know some doctors don’t consider ECP to be MIGS, but I believe it fits the description of a MIGS procedure quite well.) It doesn’t get the attention that the more recent MIGS procedures get; they’re new and exciting and have company reps promoting them, while ECP has been around for 10 or 12 years. Nevertheless, the clinical data show that it has efficacy and a favorable side-effect profile. In addition, it’s reusable and doesn’t leave any hardware inside the eye, and it can be used in a large number of patients. In particular, it can be used as an adjunctive treatment, because it combines well with other MIGS. Most MIGS increase outflow; ECP helps to decrease inflow, making them symbiotic.
One thing we have to be concerned about with ECP is IOP spikes. Two separate studies found almost the same incidence of IOP spikes associated with ECP. A 2010 study involving 368 eyes with at least two years of follow-up reported an IOP spike incidence of 14.1 percent.6 A study conducted by the ECP Collaborative Study Group involving 5,824 eyes with a mean follow-up of 5.2 years found an IOP spike rate of 14.5 percent. The data were reported at the American Society of Cataract and Refractive Surgery Symposium on Cataract, IOL and Refractive Surgery in San Diego in May 2007.
To minimize the likelihood of an IOP spike following ECP, it’s important to understand the etiology of the problem. There are four main reasons for an IOP spike in this situation: excessive inflammation; retained viscoelastic; excessive treatment; and steroid response. Let’s discuss each of these in detail.
• Inflammation. ECP causes some coagulative changes to the tissue, and that incites an inflammatory response. This can be addressed prophylactically, both before and after the procedure. Postoperatively, I always perform a subconjunctival injection of dexamethasone (Decadron), and I slowly taper the topical steroids over a month to minimize any severe inflammatory response. If the patient has significant inflammation or fibrin on postoperative day one, I can augment that with oral steroids for five to seven days. That usually gets rid of any excessive anterior chamber inflammation. You can also transiently increase the topical glaucoma drops regimen and/or add some oral carbonic anhydrase inhibitors such as acetazolamide or methazolamide.
• Retained viscoelastic. This is probably the most common cause of a postoperative IOP spike. When performing ECP, we have to inflate the ciliary sulcus with viscoelastic to give the probe room to reach the ciliary processes. Then, at the end of the case, we have to be very mindful to remove all of the viscoelastic, not just from the anterior chamber (as we usually do) but also from the ciliary sulcus. If I have any concern that there may still be viscoelastic left in the sulcus, I’ll go in with a cannula and manually inject BSS into the sulcus to make sure I’ve cleared the sulcus of all viscoelastic.
On postoperative day one, if the IOP is elevated and I believe that residual viscoelastic is the problem, I’ll burp the wound, being sure to place a drop of ophthalmic betadine on the area first to minimize the risk of infection. If I’ve done this two or three times within 24 hours and the pressure still hasn’t gone down enough, and extra glaucoma medications don’t seem to be helping, I’ll consider taking the patient back to the OR to remove residual viscoelastic.
• Pigmentary debris from over-treatment. Retained pigmentary de-bris, caused by treating ciliary tissue until it makes an audible pop, can lead to IOP spikes. The popping sound indicates that tissue has exploded, releasing pigment; the pigment de-bris can then block the trabecular meshwork and cause a rise in IOP.
Obviously, the best way to deal with this is by preventing the explosions in the first place, which can be done by titrating power appropriately. (See the tips on p. 25.) However, if you do cause popping, you’ll need to spend a fair amount of time performing irrigation and aspiration to clear out the debris. If that happens, you should be aggressive with postoperative steroids; you can give subconjunctival dexamethasone and/or increase the frequency of postoperative topical steroids.
Usually, the pressure will return to normal (or even lower) within five to seven days. Needless to say, prevention is the better approach.
• Steroid response. IOP can also spike in response to steroid use. Obviously we’re being aggressive with steroids after ECP to try to decrease any inflammatory response. As you know, some glaucoma patients are steroid responders. If your patient has a history of steroid response, don’t overtreat, and avoid using stronger steroids like difuprednate (Durezol).
When I’m treating a steroid responder, I’ll use my regular postoperative regimen—prednisolone four times a day. Sometimes I’ll use loteprednol instead of prednisolone, but I’ll often augment it with postoperative topical NSAIDs to minimize the postoperative inflammatory response. I taper the patient off the topical steroids as soon as possible.
Other less-common causes of pressure spikes following ECP include:
• Aqueous misdirection. Aqueous misdirection, or malignant glaucoma, is extremely rare in this situation—I’ve only seen one case in my career—but it can occur following any type of intraocular surgery, including ECP. Unfortunately, it’s easy to miss because we typically consider aqueous misdirection as a process only seen after trabeculectomies or tube shunt implantation. We need to remember that if the patient develops a shallow anterior chamber and elevated IOP postoperatively, aqueous misdirection is a possible etiology.
• Cystoid macular edema. CME is uncommon following CPC, although it’s not as rare as aqueous misdirection. It probably occurs in fewer than 1 percent of patients. Nevertheless, CPC incites a post-operative inflammatory reaction, so the patient has some risk of developing CME—especially if the patient is diabetic. You want to stymie any potential postoperative inflammation as much as possible to minimize this risk.
I minimize the risk by treating prophylactically with subconjunctival dexamethasone, and when I’m going to perform ECP, I treat both preoperatively and postoperatively with topical NSAIDs. If CME does develop postoperatively, I’ll increase the topical steroid regimen and I’ll seriously consider using sub-Tenon’s steroids to ameliorate the edema.
 |
|
|
Micropulse CPC
The latest iteration of CPC, micropulse cyclophotocoagulation, or MPCPC, minimizes the elevation of temperature inside the targeted tissue by dividing a continuous-wave beam of energy into a series of short pulses. As a result, the targeted tis-sue cools back down before any coagulation takes place. This has the effect of altering the tissue without destroying it. For that reason, I like to refer to this as “cyclomodification,” rather than cyclodestruction.
This lack of destruction has been demonstrated in several studies. One study reported by Murray Johnstone, MD, at the 2017 meeting of the American Glaucoma Society, found that tissue treated with MPCPC showed no sign of ciliary epithelium coagulative damage or motion. Only subtle changes could be seen upon evaluation.
 |
This raises an interesting question: The traditional assumption about the mechanism of action of CPC has been that it causes damage that prevents the ciliary processes from creating aqueous, thus helping to lower pressure by lessening “input” rather than enhancing outflow. If this version of CPC isn’t causing any visible damage, why does intraocular pressure drop after treatment?
The answer may lie in an older study published in 1994.7 This study found that TSCPC causes enlargement of the extracellular space in the stroma and separation of the ciliary muscles from the sclera. This suggests that the decrease in IOP may have resulted from both reduced aqueous secretion and enhanced uveoscleral outflow. Given that MPCPC minimizes dam-age to the tissue, this may explain why it’s nonetheless effective at reducing IOP.
Here are some pearls for making the most of MPCPC:
• Don’t hold the P3 Probe the way you’d hold the G-Probe. Occasionally, surgeons report a lack of good response to treatment with MPCPC. In my experience, this is usually the result of a simple technique problem involving how the probe is being held against the eye. Surgeons who’ve used the G-Probe to perform transscleral CPC are accustomed to placing that probe positioned at the limbus, held parallel to the visual axis. Because of the design of the G-Probe, including the off-center positioning of the laser inside the probe and the curved tip designed to hug the globe, this delivers the laser energy to the correct area inside the eye.
The P3 Probe used for micropulse CPC is designed differently. It has a more rounded tip, with the laser coming out at the center, so it should be positioned about 1 mm back from the limbus and held perpendicular to the surface of the globe in order for the laser to treat the correct area inside the eye. If it’s held the same way as the G-Probe—which surgeons may instinctively do—the treatment will have little effect. (See sample pictures, p. 26.)
The other factor that can cause a surgeon to hold the probe incorrectly is a tight orbit. If there’s not much room around the globe, it’s challenging to hold the probe perpendicular to the eye, 1 mm back from the limbus. This encourages the surgeon to hold it more like the G-Probe, with the same less-than-excellent results.
Because this misunderstanding about how to hold the P3 Probe is so widespread, when people tell me they’re not getting much response to treatment, that’s the first thing I tell them to try changing.
• When sweeping the probe along the eye, try doing shorter segments at a time. When you’re sweeping across 180 degrees, the probe tip may get stuck on the conjunctiva, particularly if the ocular surface isn’t well lubricated. I prefer sweeping across shorter 90-degree segments; I find the probe tip is less likely to get stuck, making treatment less awkward.
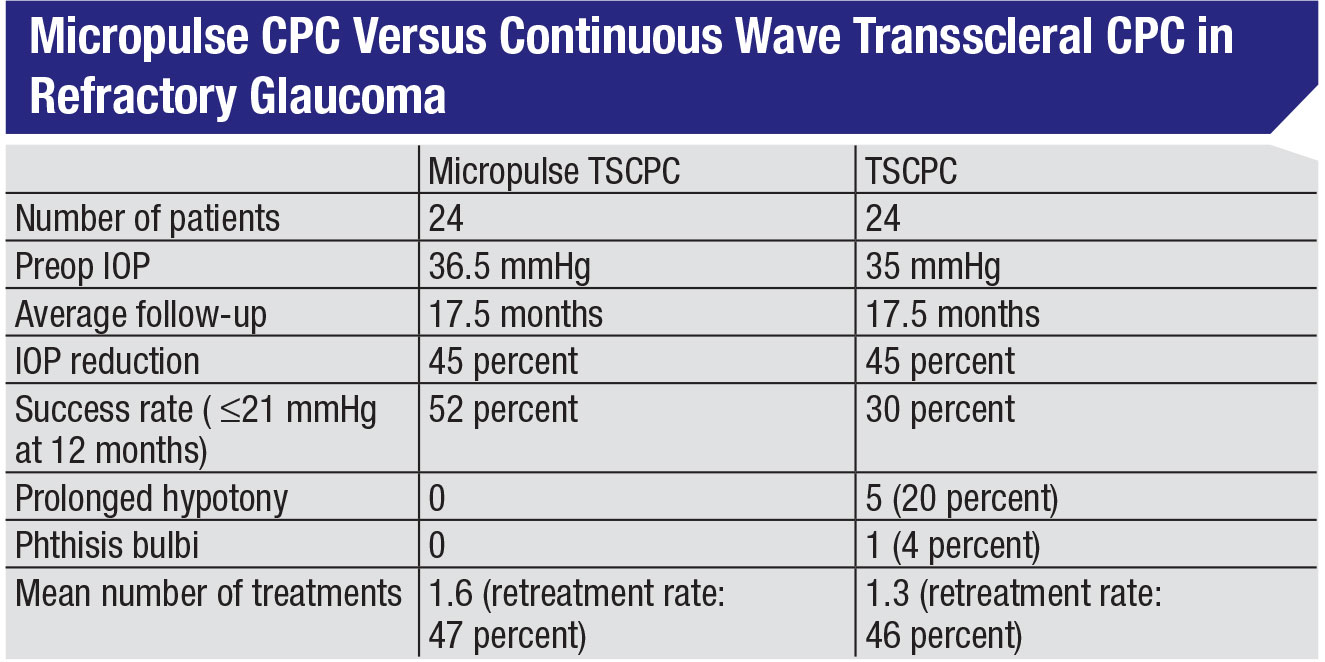
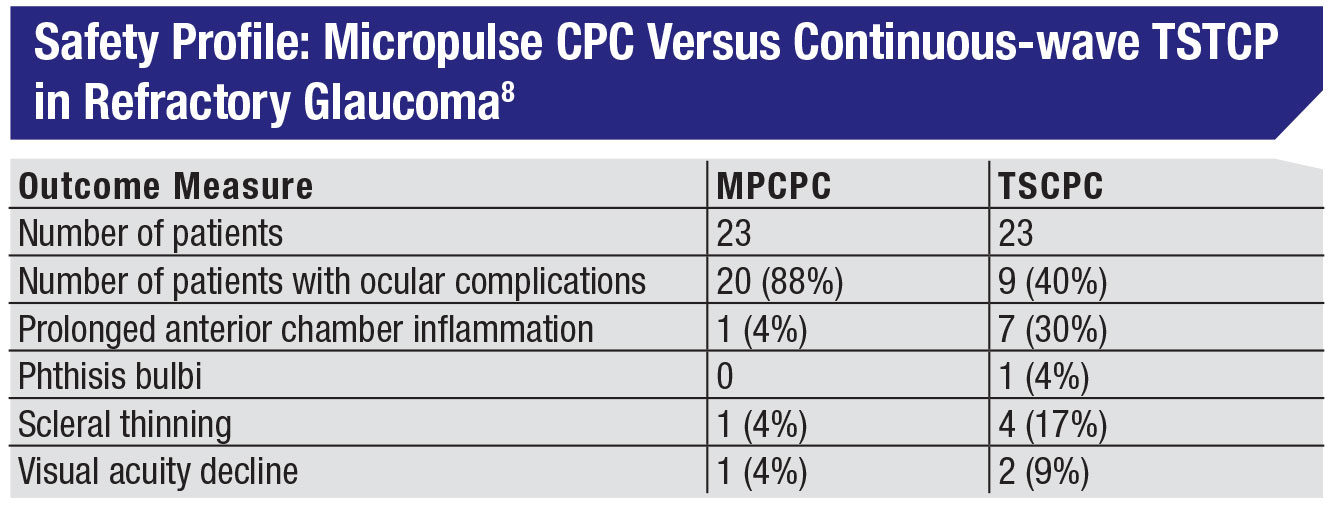
• Don’t be afraid to use MPCPC in cases of refractory glaucoma. Given that micropulse minimizes the tissue damage, surgeons may worry that it won’t be effective in refractory individuals. One excellent study looked at that, comparing micropulse CPC to traditional continuous wave diode CPC in 48 end-stage glaucoma patients.8 The groups had nearly identical IOPs before treatment: 36.5 mmHg vs. 35 mmHg, and both approaches produced a 45-percent reduction in IOP. In fact, in this study the micropulse group had a slightly greater success rate, based on the definition of success used in the study—pressures between 6 and 21 mmHg and a 30-percent reduction in IOP at 18 months. (See table, p. 23.)
The only significant tradeoff was a slightly higher retreatment rate. Here it was an average of 1.6 treatments to achieve success, vs. 1.3 treatments for the continuous wave group. (In our experience it can sometimes be more than that. In my practice the mean number of treatments is closer to two, particularly for patients with advanced disease.) So, you’re trading a little convenience for the safety and efficacy you gain with MPCPC.
| Hopefully, more ophthalmic |
Three Key Strategies
The following strategies will help minimize any complications when performing CPC:
• Avoid causing the popping sound. The audible “pop” you may hear during TPC or ECP is the sound of tissue exploding. Exploding tissue is never a good thing, so this is something you want to avoid. (You can see ciliary processes popping—on camera—in a video by Craig Chaya, MD’s group in Salt Lake City, at youtube.com/watch?v=OKMR3tCenlk. The footage of ciliary processes popping starts about 2:45 into the video.)
There are two protocols for avoiding causing the ciliary processes to explode. Historically, surgeons started between 1,750 and 2,250 mW of energy applied for 2,000 mS, and would turn up the power until a little crackle was audible; at that point, you’d back off the power a little bit and proceed with treatment. I would typically perform 12 spots of treatment per hemisphere, skipping the 3 o’clock and 9 o’clock positions (see the explanation below).
However, in the past couple of years I’ve been using the Gaasterland “slow coagulation technique” which involves starting with a lower power and applying it over a longer duration. Again, I increase power until I hear a little crackle and then treat using slightly less than that amount. This method has resulted in a higher degree of efficacy with a better side-effect profile: less edema; less inflammation; and less discomfort postoperatively. (In either technique the important thing is to avoid reaching the level at which you hear a loud pop. If you hear that, you’re more likely to get significant postoperative inflammation, fibrin and possibly even hypotony.)
• Avoid performing CPC at the 3 and 9 o’clock positions inside the eye. The long, posterior ciliary vessels, including the arteries, typically enter the eye at the 3 and 9 o’clock positions. For that reason, performing CPC at those locations means a higher risk of causing ischemia, hypotony and all of the other negative sequelae that we’re concerned about.
Many surgeons are aware of this and try to avoid these areas when treating, but if the eye has been anesthetized using a retrobulbar block it may cyclorotate, changing the lo-
cation of 3 and 9 o’clock. (Even with-out a block, eyes may cyclorotate a little when the patient lies down.) For that reason, I mark the eye at 3 and 9 o’clock preoperatively, to avoid inadvertently treating those areas.
Although I’ve seen videos in which surgeons using micropulse CPC don’t skip the 3 and 9 o’clock sections, I still believe that it’s a good idea to skip these locations, even with this less-destructive technology. Theoretically the laser doesn’t penetrate as deeply, and it might not hit those deeper vessels, but I’m not going to take that chance. I don’t think skipping two clock hours of treatment is going to have a major impact on the outcome, so I err on the side of caution.
• When using ECP, be aware that inaccurate placement of the treatment can lead to a refractive shift. Not many surgeons realize it, but if you laser the ciliary processes a little too anteriorly, the tissue changes can cause forward movement of the iridozonular complex, resulting in a small myopic shift. Likewise, if you apply the laser a little too posteriorly, the tissue changes can pull the complex posteriorly, causing a small hyperopic shift. If this is happening, it’s likely to be a consistent problem, resulting in a consistent refractive shift one way or the other.9
Given that we still don’t get about 10 percent of our patients within 0.5 D of our target, it’s easy to overlook asymmetric ECP treatment as a possible explanation for a slight, consistent refractive surprise. However, if you’re performing EPC, there could be a connection. So, try to perform a uni-form, diffuse application across the ciliary processes—not too anterior or posterior. Then, be sure to track your refractive outcomes postoperatively and adjust your technique accordingly if you find that your refractive results are a little bit off.
 |
|
|
A Valuable Option
Today, doctors treating glaucoma are more comfortable using CPC earlier in the treatment course than they were in the past. I think this is true for two reasons: First, surgeons younger than 45 have seen their attendings use CPC earlier in the course of the glaucomatous disease process, with good results. Thus, they feel more comfortable incorporating it earlier in their treatment paradigm. The second factor increasing the use of CPC is the arrival of micropulse CPC. This has shifted the risk/benefit ratio even more in the direction of safety, and that makes surgeons more comfortable about using CPC earlier in the game.
Hopefully, more ophthalmic surgeons will realize the advantages of this approach to lowering IOP—the avoidance of patient adherence issues, the cost-effectiveness, and the benefits for the patient’s quality of life, to name a few. In terms of outcomes, close attention to detail, as well as taking advantage of some of the pearls provided here, should prevent untoward complications and ensure excellent results. REVIEW
Dr. Kammer is an associate professor of ophthalmology at Vanderbilt Eye Institute in Nashville, Tennessee. He has consulted for Allergan, Aerie, Iridex and New World Medical.
1. Eid TE, Katz LJ, Spaeth GL, Augsburger JJ. Tube-shunt surgery versus neodymium:YAG cyclophotocoagulation in the management of neovascular glaucoma. Ophthalmology 1997;104:10:1692-700.
2. Ramli N, Htoon HM, Ho CL, Aung T, Perera S. Risk factors for hypotony after transscleral diode cyclophotocoagulation. J Glaucoma 2012;21:3:169-73.
3. Quigley HA. Improved outcomes for transscleral cyclophotocoagulation through optimized treatment parameters. J Glaucoma 2018;27:8:674-681.
4. Egbert PR, Fiadoyor S, Budenz DL, Dadzie P, Byrd S. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol 2001;119:3:345-50.
5. Semchyshyn TM, Tsai JC, Joos KM. Supplemental transscleral diode laser cyclophotocoagulation after aqueous shunt placement in refractory glaucoma. Ophthalmology 2002;109:6:1078-84.
6. Lima FE, Carvalho DM, Avila MP. Phacoemulsification and endoscopic cyclophotocoagulation as primary surgical procedure in coexisting cataract and glaucoma. Arq Bras Oftalmol 2010;73:5:419-22.
7. Liu GJ, Mizukawa A, Okisaka S. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Res 1994;26:2:65-79.
8. Aquino MC, Barton K, Tan AM, Sng C, Li X, Loon SC, Chew PT. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol 2015;43:1:40-6.
9. Sheybani A, Saboori M, Kim JM, Gammon H, Lee AY, Bhorade AM. Effect of endoscopic cyclophotocoagulation on refractive outcomes when combined with cataract surgery. Can J Ophthalmol 2015;50:3:197-201.



