 |
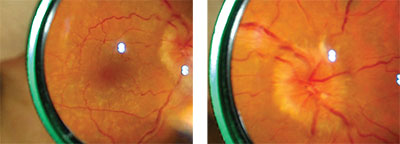
| Figure 1a (left). Mydriatic fundus photo of cryptococcal meningitis with focus on the posterior pole and macula captured with a 20-D lens and smartphone camera. By capturing video instead of still photos, the best image for highlighting the pathology of interest can be selected.4 Figure 1b (right). Mydriatic fundus photo of optic-disc edema secondary to cryptococcal meningitis, captured with a 20-D lens and smartphone camera. |
Advancements in microfabrication, optics, digital sensors and image processing have led to progressively smaller, more portable and more powerful imaging devices. Integrating these devices into wireless networks facilitates secure image transmission for tele-ophthalmology applications. As a result, there are now numerous ways to image the retina. Here, we’ll discuss a few of them.
Smartphone Ophthalmoscopy
The smartphone camera is the most ubiquitous technology available for ophthalmic imaging, and it can approach the optical quality and resolution of many commercial digital single-lens reflex cameras.
In 2013, the iExaminer (Welch-Allyn; Skaneateles Falls, N.Y.), an adaptive device used to capture fundus images with the iPhone became available. iExaminer captures images through undilated pupils; however, the quality and restricted field of view of this device has limited its use.
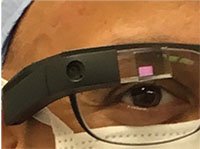 |
| Figure 2. A surgeon wearing the Google Glass, which displays the recorded image in front of the user’s eye through a prism. Google Glass has a computerized central processing unit, touchpad display screen, microphone and high-definition camera. It records in 720p with 5 MP still shots and is worn like a conventional pair of glasses. |
Another device, D-Eye (D-Eye; Pasadena, Calif.), was developed in Italy and can also be used to capture non-mydriatic fundus images. A prospective, cross-sectional study of dilated eyes demonstrated 55- to 89-percent sensitivity for detection of diabetic retinopathy using the D-Eye device fitted to an iPhone 5 compared to slit lamp biomicroscopy. Differences in sensitivity rates varied depending on the degree of retinopathy.3
All three of the above devices are bundled with associated secure HIPPA-compliant apps for image transmission; however, only the iExaminer and D-Eye are approved by the Food and Drug Administration. PEEK Retina is currently seeking FDA approval.
Taking Pictures
With practice, a smartphone camera can be used to obtain high-quality images of the posterior pole through a dilated pupil.
PEEK Vision’s CEO Andrew Bastawrous, who is also an ophthalmologist, developed a way to capture mydriatic fundus images using a smartphone.4 With a smartphone’s native camera application set to video mode with the flash “on,” the photographer holds the phone in one hand and a 20- or 28-D lens in the other in a manner analogous to performing indirect ophthalmoscopy. By capturing video rather than still photos, frames that best highlight the pathology of interest can be selected (Figure 1a and 1b). This technique is most useful in resource-poor areas and in inpatient settings.
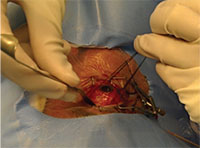 |
| Figure 3. A still photo captured with the Google Glass during a scleral buckle surgery. Photo capture is activated with a head tilt and voice command from the surgeon. This is the surgeon’s point of view when scleral quadrants are being examined prior to buckle placement. |
In the Smartphone Ophthalmoscopy and Reliability Trial, images obtained via smartphone ophthalmoscopy detected 74.3 percent of critical fundus findings (like retinal hemorrhage, subretinal fluid and optic disc edema) found on binocular indirect examination.5 This rate was only slightly less than the 77.1 percent detection rate for critical fundus findings with a standard fundus camera. Multiple studies have validated the utility and quality of images captured using this technique,5,6,7 though the need for dilation and a learning curve similar to that of indirect ophthalmoscopy may limit its utility for non-ophthalmologists. Other investigators have created and tested adapters to perform head-mounted indirect ophthalmoscopy using an iPhone or GoPro camera (GoPro; San Mateo, Calif.).8,9
A group from Stanford University has tried to simplify this indirect technique by using a smartphone mount to hold a 28-D lens (Paxos, Digisight Technologies; San Francisco). This might limit the bimanual variability that occurs with indirect ophthalmoscopy. Two studies demonstrated that this device is capable of producing high-quality fundus images that can be useful in screening for moderate to more advanced diabetic retinopathy.10,11
Surgeon POV Recording
It’s been hard to obtain surgical videos of globe and ocular adnexa from the surgeon’s point of view, a perspective that helps teach surgical techniques to others. Operating microscopes obtain high-quality videos of the anterior and posterior segment, but are hampered by a static, top-down point of view. A videographer can record surgery, but an off-axis perspective and non-optimal lighting limit these videos. Surgical cameras mounted in the overhead lights work well but can be cost-prohibitive.12 Recently, relatively affordable first-person cameras such as Google Glass and GoPro have been used effectively to record eye surgery from the surgeon’s point of view.13,14
 |
| Figure 4. The GoPro Hero4 Silver Edition. GoPro is worn on the forehead of the surgeon with the camera mounted on a head strap within a case. The compact camera is equipped with a touchscreen display, micro-speaker, and Wi-Fi. It records in 1080p and takes 12 MP still shots. |
The GoPro (Figure 4), designed to record extreme sports, features a high recording frame rate and dynamic lighting adaptability; it has been used to record a tap-and-inject procedure for endophthalmitis.16 The GoPro device can also be used hands-free to record photos and videos in the operating room (Figure 5). Simultaneous recording with two head-mounted GoPro cameras has also been used to record 3D video for stereoscopic viewing of scleral buckle surgery.17
Each device has some advantages. Google Glass is comfortable to wear and looks like a pair of glasses. The surgeon can see what’s being recorded in real time, so adjustments in camera position are easy to make. However, the camera doesn’t have a great dynamic range, and the spotlights in the operating room can overexpose the image. The GoPro camera has better dynamic range and comes with easy-to-use software that facilitates post-production zoom and adjustment of both audio and video quality. With the GoPro, the image can be sent to a smartphone so an assistant can watch the live footage. The surgeon, however, can’t see what’s being recorded in real time and, since the surgeon continuously moves, the forehead-mounted camera also moves. This means that the surgical field is frequently out of the frame. To increase the likelihood of capturing useful images with GoPro, simultaneous recording with two GoPro cameras, one on the head and another on the chest, has been proposed,18 or the assistant viewing the smartphone with live feed can direct the surgeon to keep the image usable.
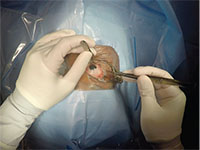 |
| Figure 5. Still photo of scleral buckle surgery captured with GoPro Hero4 Silver. GoPro can be controlled via live stream onto a nearby smartphone or computer. This is the surgeon’s point of view, showing the scleral buckle being placed around the eye. |
It’s clear that portable ophthalmic imaging will become increasingly important in the years to come. In addition to using video equipment for recording procedures for presentations and lectures, tele-ophthalmology networks are growing both in developed and developing countries, and portable, easy-to-use, low-cost, high-resolution panophthalmic imaging is critical. Although no current portable device ideally images the anterior and posterior segments, there are several options currently available, and newer ones are in development. Additionally, first-person surgical videography offers a new and potentially better way to educate others on surgical technique, and the technology continues to evolve to help us to meet the needs of patients and our profession. REVIEW
Melissa Sieber, MD, is a second year ophthalmology resident at Wills Eye Hospital. Murtaza Adam, MD, is a second year retina fellow at Wills Eye Hospital. Sunir Garg, MD, is a professor of ophthalmology at MidAtlantic Retina, the retina service of Wills Eye Hospital. He can be reached at sgarg@midatlanticretina.com. The authors have no financial interest in any of the products discussed.
1. Bastawrous A, Giardini M, Bolster NM, et al. Clinical validation of a smartphone-based adapter for optic disc imaging in Kenya. JAMA Ophthalmol 2016;134:151–158.
2. Garg SJ. Applicability of smartphone-based screening programs. JAMA Ophthalmol 2016;134:158–159.
3. Russo A, Morescalchi F, Costagliola C, et al. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading diabetic retinopathy. Am J Ophthalmol 2015;159:360.
4. Bastawrous A. Smartphone Fundoscopy. Ophthalmology 2012;119:432-433.
5. Adam MK, Brady CJ, Flowers AM, et al. Quality and diagnostic utility of mydriatic smartphone photography: The smartphone ophthalmoscopy reliability trial. Ophthalmic Surg Lasers Imaging Retina 2015;46:631-637.
6. Ryan ME, Rajalakshmi R, Prathiba V, et al. Comparison among methods of retinopathy assessment (CAMRA) study. Ophthalmology 2015;122:2038-2043.
7. Haddock LJ, Kim DY, Mukai S. Simple, Inexpensive technique for high-quality smartphone fundus photography in human and animal eyes. J Ophthalmol 2013. doi: 10.1155/2013/518479
8. Wang A, Avallone J, Guyton DL. Head mounted digital camera for indirect ophthalmoscopy. Invest Ophthalmol Vis Sci 2014;55:1606-1606.
9. Welch RJ, Nguyen QD. A novel approach to ophthalmic photography using a portable and versatile camera device. Invest Ophthalmol Vis Sci 2015;56:4102-4102.
10. Ludwig CA, Murthy SI, Pappuru RR, et al. A novel smartphone ophthalmic imaging adapter: User feasibility studies in Hyderabad, India. Indian J Ophthalmol 2016;64:191-200.
11. Toy BC, Myung DJ, He L, et al. Smartphone-based dilated fundus photography and near visual acuity testing as inexpensive screening tools to detect referral warranted diabetic eye disease. Retina 2016;36:1000-1008.
12. Matsumoto S, Sekine K, Yamazaki M, et al. Digital video recording in trauma surgery using commercially available equipment. Scand J Trauma Resusc Emerg Med 2013;21:27.
13. Hashimoto DA, Phitayakorn R, Fernandez-del Castillo C, Meireles O. A blinded assessment of video quality in wearable technology for telementoring in open surgery: The Google Glass experience. Surg Endosc 2016;30:372-378.
14. Paro JAM, Nazareli R, Gurjala A, et al. Video-based self-review: Comparing Google Glass and GoPro technologies. Ann Plast Surg 2015;74:S71-S74.
15. Rahimy E, Garg SJ. Google glass for recording scleral buckling surgery. JAMA Ophthalmol 2015;133:710-711.
16. Calvo CM, Sridhar J, Hong BK, et al. Video recording of vitreous tap and intravitreal antibiotic injection from the surgeon’s perspective. Retina 2015;35:2147-2149.
17. Birnbaum FA, Wang A, Brady CJ. Stereoscopic surgical recording using GoPro cameras: A low-cost means for capturing external eye surgery. JAMA Ophthalmol 2015;133:1483-1484.
18. Warrian KJ, Ashenhurst M, Gooi A, Gooi P. A novel combination point-of-view (POV) action camera recording to capture the surgical field and instrument ergonomics in oculoplastic surgery. Ophthal Plast Reconstr Surg 2015;31:321–322.



