Perhaps you didn’t pay enough attention to the retina before implanting a premium IOL, got fooled by a patient’s masked corneal astigmatism, committed to a wound size that was too small, struggled with small pupils or made an intumescent cataract burst from an ill-advised approach. If any of these problems sound familiar—or if you don’t want them to become familiar—follow these five tips to steer clear of trouble.
1. Don’t forget the retina.
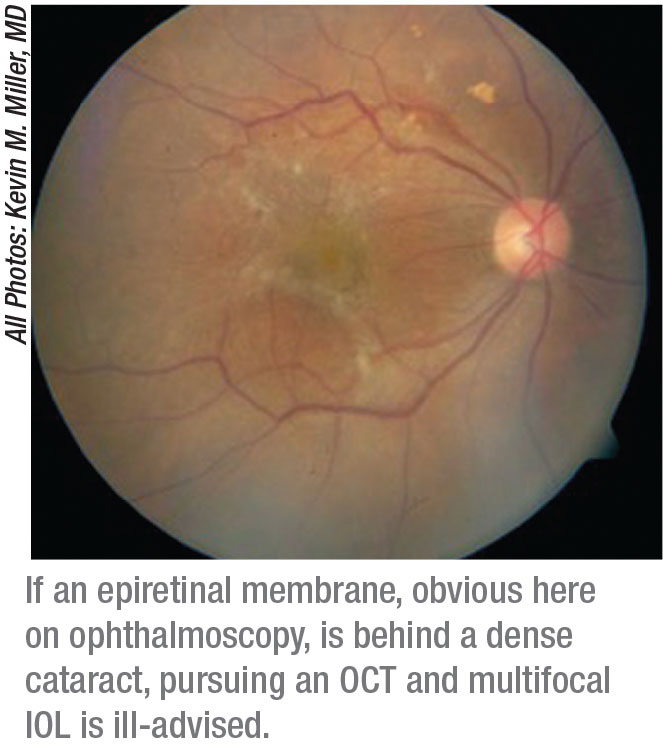 |
“It should go without saying that if you can’t see the back of the eye, you’re not going to implant a premium IOL because patients won’t be happy with their vision afterward,” says Kevin M. Miller, MD, Kolokotrones Chair in Ophthalmology at the University of California, Los Angeles. “You also need to obtain an OCT before considering a premium IOL.”
Below are some of the conditions that Dr. Miller says should rule out the use of these lenses:
• vitreomacular traction;
• epiretinal membrane;
• full-thickness macular holes;
• severe macular degeneration; and
• pigment epithelial detachments close to the center of the fovea.
“Remember, also, that if you’re planning to implant a premium IOL, you need to examine the retinas of both eyes, because eventually both eyes must be acceptable for the premium lens,” says Dr. Miller. “If you need to do regular cataract surgery, you’ll want to do an ultrasound to also rule out pathology in the retina, such as a mass, a tumor or a detached retina.”
Kathryn M. Hatch, MD, director of the refractive surgery service at Massachusetts Eye & Ear and an assistant professor of ophthalmology at Harvard Medical School, agrees that implanting premium IOLs in the presence of AMD isn’t worth the risk. “I worry about degrading the patient’s vision,” she says. “If the patient’s vision gets worse after you put in a multifocal, that could create some serious unwanted issues.”
Alan S. Crandall, MD, senior vice-chair, Department of Ophthalmology & Visual Sciences at the University of Utah’s Moran Eye Center, orders an OCT on every cataract surgery candidate. “I often pick up on an epiretinal membrane that’s pretty hard to see without an OCT scan. I think it’s important to capture any findings that may affect a patient’s surgery and his postop vision in the future. And it helps me set reasonable postop expectations for my patient,” he says.
2. Manage cases of astigmatism realistically.
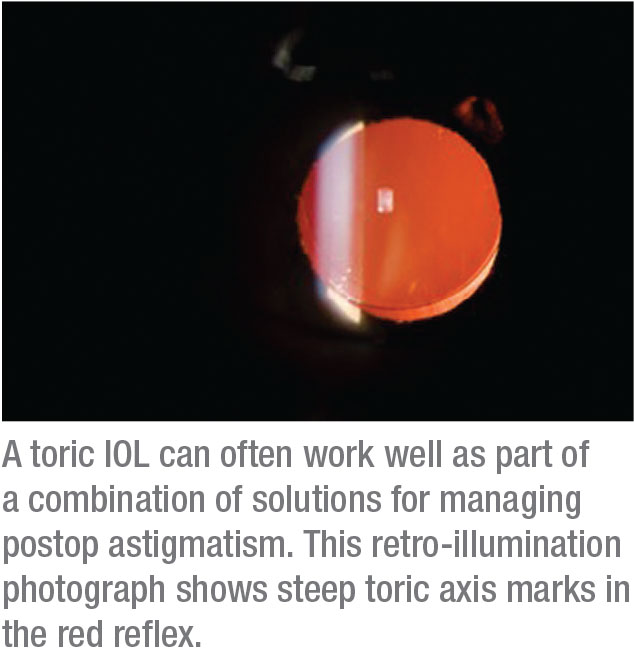
Dr. Hatch says she analyzes corneal topography—and more—on every preop patient by using an OPD-Scan III (Marco/Nidek). “I’m checking for pathology and astigmatism,” she notes. “We’ll diagnose irregular astigmatism or keratoconus in patients who didn’t know they had these issues. This is critical if your goal is to reduce spectacle dependence after surgery. Even three-quarters or a half of a diopter of astigmatism after surgery can really degrade the quality of vision. We keep all options available, including limbal relaxing incisions or a toric IOL.”
Mindful of patients’ frequent disappointment over astigmatism, Dr. Miller tries to improve it as much as he can. “My cut-off is 20/200 or better,” he says. “They must have the potential to see the big E on the eye chart after surgery. You can do limbal relaxing incisions, phaco incisions and use a toric lens. Even if the potential visual gain isn’t great, or if the patient has a small epiretinal membrane or a little drusen, it can be worth the effort.”
Dr. Miller recommends using the words “manage” instead of “correct” astigmatism, noting that “we surgeons can’t make everybody better. Sometimes, we’re going to deliberately make them worse to give them the best long-term result.”
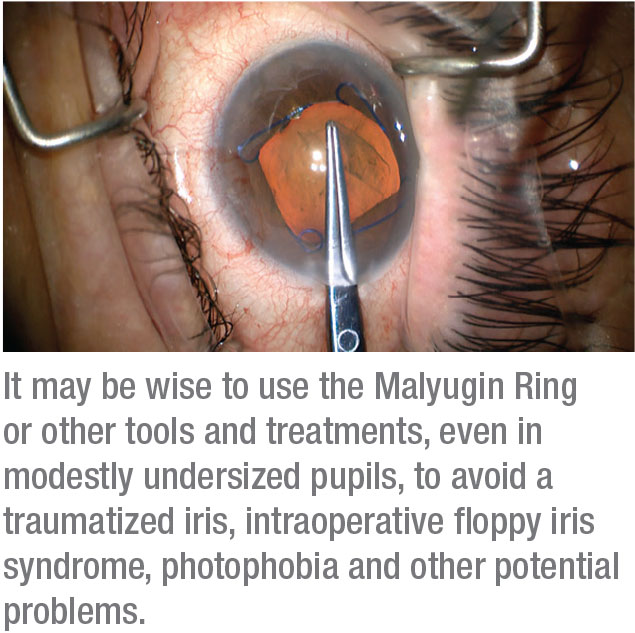 |
For example: Your patient has no astigmatism preop, but certainly will postop. “So what can you do?” asks Dr. Miller. “You can make a temporal incision and their postop astigmatism will be vertical. Or you can create a superior incision and make the astigmatism horizontal. Let’s say it’s 0.3 D vertical or 0.3 D horizontal. The vertical residual astigmatism will actually go to zero over time. The 0.3 D residual horizontal is going to get worse, going from 0.3 D, to 0.4 D, to 0.5 D and worse. So there’s a best approach, which is to make a small temporal incision, with-the-rule, vertically. This will yield the best long-term result, even though the patient is worse in the beginning.”
Dr. Miller recalls a patient who had no astigmatism in his prescription but 2.5 D of astigmatism in the cornea that wasn’t initially apparent. “I took out the lens and I implanted a spherical IOL, unaware of the astigmatism in the cornea,” he says. “All of a sudden, this patient who had no history of astigmatism had 2.5 D of astigmatism that had been masked by lenticular astigmatism. The patient never needed a correction for astigmatism in his glasses and now I had to put in a high-powered toric lens. That’s a difficult conversation to have.”
Dr. Miller recommends remaining mindful of the limitations of astigmatism management—and the interventions that can maximize results, including:
• a phaco incision on the steep axis to cut down on astigmatism by 0.2 D to 0.5 D;
• a toric IOL; and/or
• limbal relaxing incisions, including after toric implantation if more than 4 D of astigmatism correction is needed.
“After surgery, if the patient still has residual astigmatism, I wouldn’t try to rotate or change the lens,” he adds. “Laser refractive correction can address residual cylinder.”
3. Ensure appropriate wound size.
“Your incision can’t be too tight, or too loose,” says Dr. Miller. “If you make it too tight, you end up having to stretch it to get the phaco tip and lens inside the eye and the wound will leak. These are the incisions you end up needing to put a stitch into. This can become very counterproductive.”
Dr. Hatch says she generally doesn’t vary the size of her incisions, unless she enlarges one to implant a large IOL or to use a different cartridge. “This happens only occasionally,” she adds.
 |
Dr. Crandall explains that he always measures before he makes his incision. He chooses his wound size based on the spherical aberrations of the cornea, lens power (in most cases) and cartridge size.
“You want your wounds to be reproducible,” he says. “I always calculate based on the sizes of different lenses, which will determine the size of my incision. If you don’t measure, you risk making an incision that’s too small, which can easily cause you to strip Descemet’s membrane.”
4. Manage small pupil size.
Confronting the small pupil is a regular occurrence for Dr. Crandall. “Because a lot of folks here in Utah were originally from Sweden, Norway and Denmark, you could say I live in the land of pseudoexfoliation,” he says dryly. “Just about everybody’s pupils are small. As a result, I’ve become a big believer in managing the pupil. I use the Malyugin Ring 2.0 (MicroSurgical Technology), the capsule expander (XpandNT Iris Speculum, Diamatrix) and the MST capsule hooks (MicroSurgical Technology). I have three or four different devices I use, depending on the rigidity of the pupil.” Other devices that can be applied in these situations include the Iris Expander (Oasis Medical) and the I-Ring (Beaver-Visitec International).
Dr. Crandall recommends making sure your patient’s pupil size is at least 5 mm. “There’s no question that I can do cataract surgery through a 3-mm pupil,” he says. “But the cost of doing that is increased inflammation. You’re needlessly moving the pupil. In some respects, you’re going back 15 years, when we weren’t so elegant in these cases and we often left cortex behind.”
Despite today’s tools and treatments for safely managing small pupils, surgeons note that this anatomical challenge can too easily lead to a traumatized iris, intraoperative floppy iris syndrome, photophobia and other potential problems. When she sees a small pupil, Dr. Hatch prepares for worst case scenarios. “If a patient has a small pupil in the office,” she says, “I know I may need to use a Malyugin Ring or iris hooks or mydriatic agents.” These include:
• phenylephrine 1% and ketorolac 0.3% (Omidria);
• tropicamide (Mydriacyl); and
• cyclopentolate (Cyclogyl).
“If a patient is on the borderline of a small pupil, I usually err on the side of caution, and just put in the ring or use a mydriatic agent,” she adds.
Besides using dilating agents, Dr. Miller says he’ll dilate a small pupil with high-viscosity (cohesive) viscoelastics, such as VisCoat (Alcon), EndoCoat (Johnson & Johnson Vision or OcuCoat (B + L). “And then, of course, you can use the rings and devices,” he says. Alternatively, some surgeons use a mixture of epinephrine 0.025%/lidocaine 0.75% in a fortified BSS solution (“epi-Shugarcaine”).
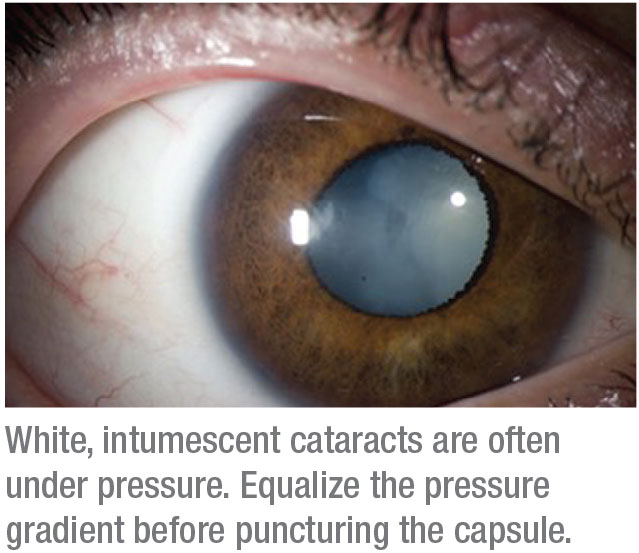
5. Approach an intumescent cataract with caution.
 |
Through the University of Utah’s Ghana Exchange Program and his role as co-director of the International Outreach Division at the John A. Moran Eye Center, Dr. Crandall removes 100 to 200 intumescent cataracts per year, performing surgeries in India, Nepal, Ethiopia, Tanzania, Ghana, South Sudan, Guatemala, Haiti and Micronesia. He’s also involved in the Himalayan Cataract Project and operates on patients in the Navajo Nation in southern Utah. He says he’s learned techniques that could help his colleagues who struggle with these cases.
“The first step is to figure out if the lens is very intumescent,” he explains. “The minute you open it up, is it going to try to split? I always stain the capsule, 100 percent of the time. I make a micropuncture centrally, maintaining the ability to immediately remove flocculant material via a cannula. The main thing is that you have to pressurize the eye so that the fluid doesn’t come out in a burst.
“What most surgeons do that’s incorrect,” Dr. Crandall continues, “is when they start their rhexis, they inadvertently hit the wounds, releasing viscoelastic, which immediately creates a fluid wave of power toward the cornea, and that’s usually what generates the burst that you don’t want. So what I do is stain the capsule and put in my viscoelastic. I don’t even make my main wound until I get a puncture in the lens and we can remove all the flocculant material. Then I make the main wound and go in through a sideport.”
When the patient has an intumescent cataract, Dr. Miller says he takes the simplest approach possible. “We just want to get in, implant a monofocal lens and get out without experiencing a big problem,” he says. “I also use a toric lens if the capsular bag is intact after cataract removal and the patient has a lot of astigmatism.”
Dr. Hatch says these cases sometimes prompt her to alter her technique, especially when she’s making a capsulorhexis and opening the capsule. Like Dr. Crandall, she uses a needle to puncture the capsule, withdrawing it in the same motion. She never uses her femtosecond laser on these cataracts. “You can’t use the laser to soften white cataracts, either, because the femtosecond laser can’t image them,” she says. “Often, the best you can do is just use a lot of trypan blue.”
Everyday Challenges
Although most cataract surgery cases go smoothly, these situations can be unsettling and rattle your confidence. Like her colleagues, Dr. Hatch says taking extra time and preparing for unusual circumstances can turn potential problems into everyday challenges and help you avoid disappointment afterward. “It’s all about thinking it through and planning your steps,” she says. “You have to have a plan.” REVIEW
Drs. Miller, Hatch and Crandall are consultants for Johnson & Johnson Vision. Drs. Miller and Crandall are also consultants for Alcon. In addition, Dr. Crandall is a consultant for ASICO, Excel–Lens, Carl Zeiss Meditec, Mastel Surgical, MicroSurgical Technology, New World Medical, Omeros and iVeena.