Despite the appearance of increasing numbers of branded OVDs, many surgeons take the use of viscoelastics, or ophthalmic viscosurgical devices, for granted when performing cataract surgery. Although the core functions of OVDs haven’t really changed, experts nonetheless point to a continuing need to better understand and embrace their complex behavior for optimal use.
Steve Arshinoff, MD, FRCSC, an associate professor in the Department of Ophthalmology and Vision Sciences, University of Toronto, and a clinical assistant professor at McMaster University in Hamilton, Ontario, Canada, says the best way to meet this need is to focus on the two basic properties of OVDs: viscosity and cohesive-dispersive behavior. “There are no OVDs that are both cohesive and dispersive; they all fit somewhere along a line from very cohesive to very dispersive” says Dr. Arshinoff, a pioneer of many OVD techniques.
In this report, Dr. Arshinoff joins other leading surgeons to help you better understand where your OVDs are along that line⎯and at what degree of viscosity⎯the next time you reach for one to undertake a specific surgical task.
Basic Functions
For basic cataract surgeries, OVDs are used to maintain space and protect tissues. “Protecting tissues also means compartmentalizing, keeping some parts of the inside of the eye in one place and other parts in a separate place during certain parts of surgery,” says Kevin M. Miller, MD, Kolokotrones Chair in Ophthalmology, University of California, Los Angeles. “At the start of surgery, you’re powering through the cataract using ultrasound. You want to protect the cornea and iris from the ultrasound and the flying fragments. Coating these tissues is the most important function of an OVD at that stage.”
Once the cataract has been removed, he continues, “space maintenance becomes a high priority. When you’re going in and out of the eye with instruments, the infusion line doesn’t provide enough support. The chamber is now at risk of collapsing. Cohesive OVDs help prevent a collapse by maintaining space. OVDs are also good for opening things up—such as the capsular bag.”
 |
|
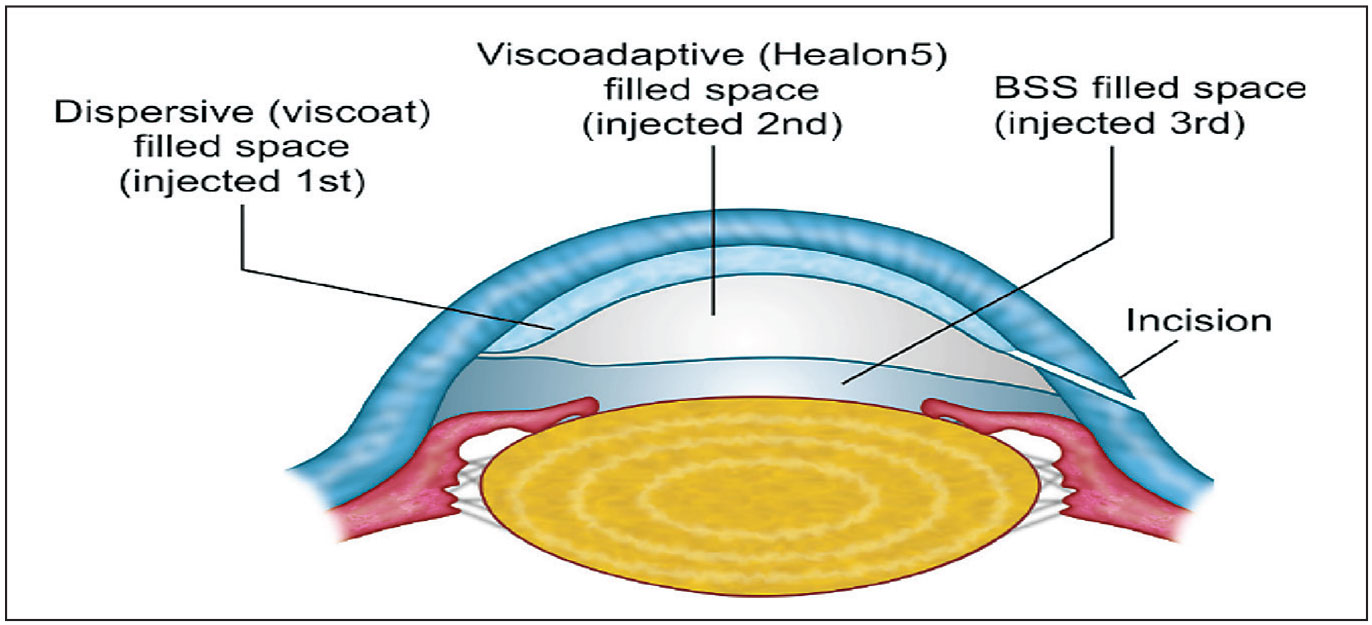
Figure 1. The layering of dispersive and visco-adaptive OVDs on top of BSS, referred to as the Tri-Soft Shell Technique (TSST), is one of several “soft shell” techniques that provide specific benefits, this one to protect the inner cornea against endothelial loss. Image courtesy of Steve Arshinoff, MD, FRCSC. |
The physical properties and characteristics of OVDs are determined primarily by the molecular chain length and concentration of their principal rheologic components, according to D. Michael Colvard, MD, who is clinical professor of ophthalmology on the volunteer faculty at Jules Stein Eye Institute, David Geffen School of Medicine UCLA, and director of the Colvard-Kandavel Eye Center in Encino, California. “These properties and characteristics also change in a predictable fashion under different conditions of fluid movement (turbulence) within the eye,” he adds.
The viscous-cohesive OVDs such as Healon GV and Healon (Johnson & Johnson Vision) and Provisc (Alcon) are long-chained sodium hyaluronate OVDs with a molecular weight of 2 to 5 million Daltons, Dr. Colvard notes. They’re referred to as “cohesive” because they cohere since their long molecular chains tend to intertwine, causing them to behave intraocularly as a cohesive unit. The advantages of cohesive OVDs are that they maintain space well, stabilize structures and enable pressurization of the space they occupy.
“A cohesive OVD will help you maintain a deep anterior chamber and prevent the incision from leaking, unless irrigation raises the intraocular pressure too high, in which case it will burp out as one piece,” adds Dr. Colvard. “In addition, cohesive OVDs are also easier to aspirate at the end of surgery, but this benefit also has a downside: In the presence of irrigation and aspiration, it means the materials won’t necessarily remain in the eye to provide a high level of endothelial protection during prolonged phacoemulsification.”
Dr. Colvard says dispersive OVDs such as Viscoat (Alcon), Endocoat (Johnson & Johnson Vision) and the new ClearVisc (Bausch + Lomb) are low-molecular weight, short-chained sodium hyaluronate OVDs (approximately 500,000 to 800,000 Daltons). Viscoat also contains low molecular weight chondroitin sulfate, which Alcon claims enhances endothelial protection. ClearVisc includes sorbitol, which Bausch + Lomb says protects the cornea against free radicals spawned by phacoemulsification.
“OVDS with low molecular weights are considered dispersives because their short molecular chains don’t become entangled as easily, allowing the OVD mass to come apart more easily,” says Dr. Colvard. “The advantage of these OVDs is that during times of high-fluid turbulence, they resist being completely washed out better than cohesive OVDs do. The downside of dispersive OVDs is that they don’t maintain space or enable pressurization as effectively as cohesive OVDs, and they’re more difficult to remove from the eye at the completion of surgery.”
When considering OVD properties, recognize the unique nature of “visco-adaptives.” They include Healon5 Pro (Johnson & Johnson Vision), composed of a higher concentration of sodium hyaluronate (2.3 percent) with a molecular weight of 3,200,000 Daltons, as well as other visco-adaptives not sold in the United States. Among those in the United States are Healon GV Pro, a super viscous-cohesive with a concentration of 1.8% sodium hyaluronate, and Healon Pro, a slightly lighter agent with a concentration of 1% hyaluronate.
 |
|
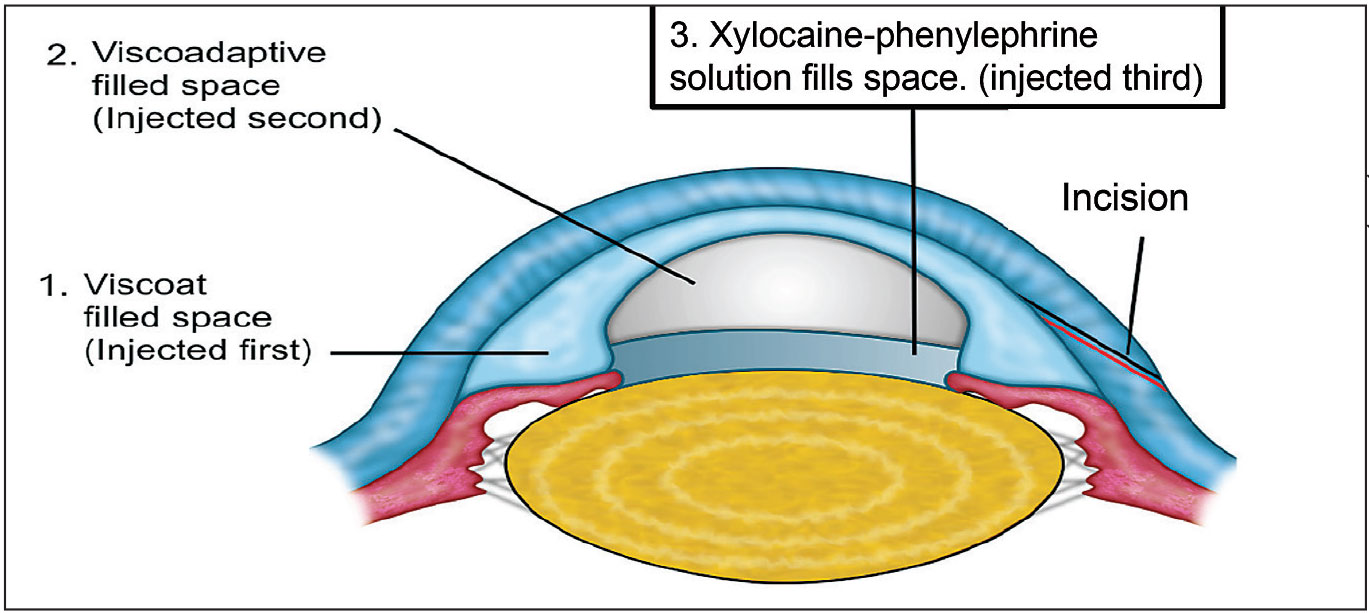
Figure 2. OVDs can be used to manage intraoperative floppy iris syndrome by employing the IFIS Soft Shell Technique (IFIS SSB), as shown above. Image courtesy of Steve Arshinoff, MD, FRCSC. |
Each contains the same length of hyaluronate chains, in different concentrations. The concentration in Healon5 is so high that it transforms an ultra-viscous cohesive at low flow rates to a fracturable solid when exposed to flow rates exceeding 25 cc per minute, according to Drs. Covard and Arshinoff. “Because of visco-adaptives’ higher sodium hyaluronate concentrations, and their behavior as fracturable solids under high flow, allowing them to become trapped behind IOLs, we need to remove visco-adaptives thoroughly to prevent postop IOP spikes,” Dr. Arshinoff says. The Ultimate Soft Shell Technique, an approach designed for visco-adaptives that’s explained below, is ideal for this use, he says.1
Two for All
Dr. Arshinoff emphasizes the importance of using cohesives and dispersives together effectively.
The most common combined use occurs when using the Soft Shell Technique, introduced by Dr. Arshinoff in the 1990s.2 “A small amount of dispersive OVD is placed on the lenticular surface and then behind that a larger amount of cohesive OVD,” he says. “The cohesive pushes the dispersive up against the cornea into a smooth layer, which has many advantages. First, the dispersive remains longer because its smooth edge doesn’t get caught by the balanced salt solution turbulence, preventing it from washing off easily. Furthermore, using a dispersive this way minimizes its irregular surface boundaries, which can cause blurring when it’s flowing. With the dispersive pressed against the cornea, you see better through a smoother layer of OVD, making surgery easier.”
The SST can help during just about all types of phaco cases, Dr. Arshinoff continues, and it’s best to use it with OVDs whose properties are as different as possible. “There is less endothelial cell loss,” he notes. “And you can pressurize the eye better if you use a dispersive with a cohesive in a structured method.”
Endothelial Dystrophies
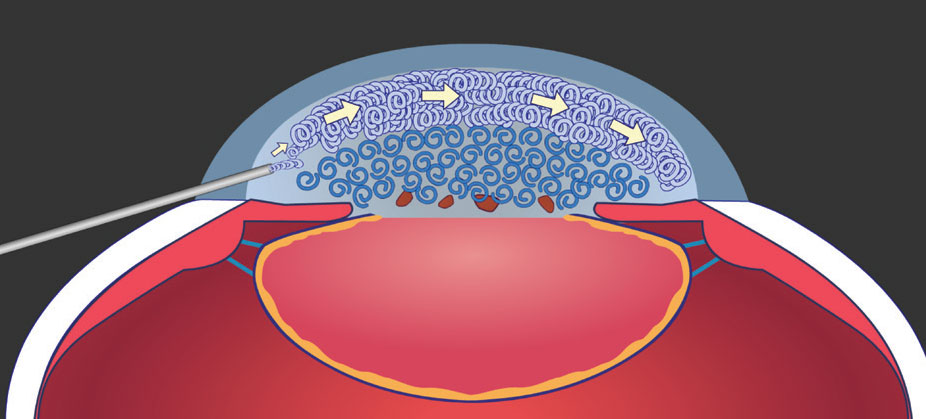 |
|
Figure 3. By directing the flow of cohesive OVD anteriorly after phacoemulsification, you can visco-dissect any dispersive OVD left in the anterior chamber away from the endothelium and toward the pupillary plane. Image courtesy of D. Michael Colvard, MD, FACS. |
Although the SST, in use for more than 20 years, is the most commonly used OVD technique in the world, Dr. Arshinoff says that it’s “not well understood” by all surgeons. “Some surgeons even say they can do cataract surgery without using any OVDs,” he notes. “Well, you can, but you will get more endothelial cell loss. This is particularly critical in patients with Fuchs’ endothelial dystrophy or other causes of decreased endothelial cell counts. When you see these patients in seven or more years, you’ll find they’ve lost more of their corneas, due to endothelial decompensation, than they would have after SST or other soft-shell techniques.”
To ensure the best long-term outcome in these cases, he also recommends lowering the flow to about 15 cc per minute. “At low-flow rates, you don’t lose the Healon5 behind the Viscoat, because Healon5, being a visco-adaptive, will act like an ultra-viscous cohesive OVD and resist aspiration at low flow rates. You’ll be operating in a very small space behind the Healon5 in the capsular bag, and nothing in the anterior chamber will move. When you finish the case, you can take out the Healon5 layer, but you can leave behind the very thin layer of the dispersive Viscoat. The postop IOP spike that may occur from leaving a small amount of OVD can be reduced with topical drops. It’s less of a concern than the cell loss you would have caused by trying to wash it off.”
Dr. Miller also uses OVDs to protect a compromised cornea, relying on dispersives. “We need to keep in mind that endothelial cells never regenerate, and we continue to lose them as we get older,” he observes. “This process accelerates as a result of trauma, surgery or inflammation inside the eye.”
During a typical cataract procedure, he continues, a patient loses 3 to 8 percent of his or her endothelial cells. “If a patient starts with a low number of cells and loses another 3, 4, 5 or even 10 percent, that can lead to swelling of the cornea, a blistering cornea or bullous keratopathy, from which the patient never recovers. That would require a corneal transplant of some sort.”
Other Uses of OVDs
Dr. Miller notes that dispersive OVDs can also help by compartmentalizing vitreous to keep it from following the phaco tip out of a surgical wound. “You can also compartmentalize lens fragments with dispersives,” he adds.
In addition, he says, a cohesive OVD can be used to expand a pupil. “This is accomplished through viscomydriasis,” he says. “For a patient who doesn’t dilate well, despite the administration of drops, you can put in a Malyugin Ring or pupil hooks, of course. But Healon5 can also be used to really open the pupil widely.”
After a capsulorhexis, while using hydrodissection, you may sometimes find you’re not able to free up a patient’s epinucleus and cortex. “Taking the path of least resistance, the fluid coming out of your cannula does a U-turn and comes right back at you,” says Dr. Miller. “Here, a cohesive or even a dispersive OVD can help by visco-dissecting all of the stuff that’s stuck.”
He notes that an OVD can also help when a capsule breaks, causing a release of vitreous. “You can put some OVD over the capsule break or in an area of a zonular compromise, compartmentalizing these structures. In the event of a capsule break, when pieces of nucleus or epinucleus start to fall, often you can get beneath those pieces with viscoelastic, performing what we call viscoelevation. You can start to fill up the vitreous cavity with the OVD and, as those pieces rise on the OVD, retrieve them before they drop onto the retina.”
Dr. Arshinoff lists other special considerations when using OVDs, such as operating on a high hyperope with a very shallow chamber. “The main thing you want to achieve for this patient is to deepen the chamber,” he says. “Using Healon5, especially with a very thin layer of balanced salt or xylocaine-phenylephrine (or adrenaline) solution behind it, adjacent to the anterior capsule (using the USST), enables you to make the rhexis much more easily.” In addition, in cases involving a compromised cornea, the Tri-Soft Shell Technique, which is a combination of the SST and USST, adds more advantages, he notes.3
Another case might involve a -20-D myope with an excessively deep chamber, making access to the nucleus difficult. “Inserting less Healon5 maintains a stable, but more shallow, anterior chamber,” says Dr. Arshinoff. “The Healon5 maintains the chamber because it blocks the incision. But the lens moves forward a bit, which you can titrate by the amount of Healon5 you inject into the anterior chamber, making it easier to create the rhexis in a high myope, instead of having to stretch back deep into the eye.”
FLACS and Other Challenges
Dr. Arshinoff says OVDs can also help prevent incomplete capsulotomies that can potentially tear out during femtosecond laser cataract surgery. Using a small cannula in front of the capsule, he says, slowly inject Healon or Healon GV until the flap moves posteriorly and becomes slightly concave.
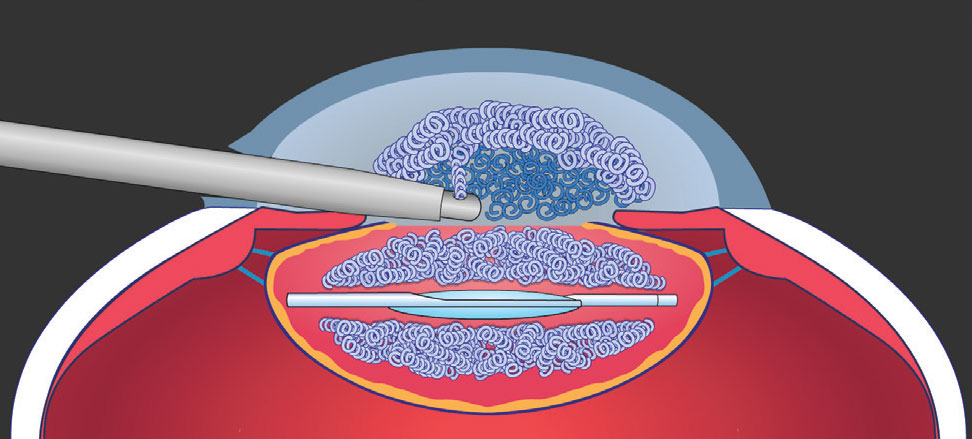 |
|
Figure 4. This maneuver places the dispersive OVD in a position in the anterior chamber where it can be more readily aspirated. This helps guard against postop IOP spikes. Image courtesy of D. Michael Colvard, MD, FACS. |
“If the flap is attached to part of the peripheral capsule, alternate injecting the OVD through the main and sideport incisions,” he says. “Because the incisions are usually 95 to 100 degrees apart, you can move the capsule back and forth with OVD injections to get it flat and slightly concave. Then use tiny forceps to pull the capsule posteriorly and toward the middle, which makes the capsule tear centrally and not peripherally, as you would risk doing by folding the flap over anteriorly to tear it.”4
For the implantation of phakic lenses, such as the EVO Visian collamer-based ICL (Staar Surgical) and the Artisan Phakic IOL (Ophtec), Dr. Arshinoff says one of the biggest challenges is removing the OVD at the end of the case without damaging the crystalline lens because of turbulence from the I/A. In these cases, he recommends using an OVD that you can wash out with gentle irrigation from a syringe. One example would be a hydroxypropyl methylcellulose solution, such as OcuCoat (Bausch + Lomb).
“If you’re using the Yamane surgical technique for a secondary IOL, consider using Healon or Healon GV,” he says. When implanting a secondary phakic IOL, he says, “remember that you have a very simple purpose for which you’re using the OVD: You want stability to transiently create space and protect adjacent structures. You need to get the lens in the eye, position it properly and come out of the eye carefully.”
Similarly, to capture an IOL in the capsulorhexis, Dr. Arshinoff urges you to be careful with your OVD choice, recommending Healon or Healon-GV so that you can wash it out more gently with a syringe and without needing to use I/A. “You also don’t want to use dispersives because they’re harder to wash out,” he adds.
Dr. Arshinoff says that most IFIS cases can be managed with an approach he calls the IFIS Soft Shell Bridge Technique, a variation of the Tri-Soft Shell Technique.6 First, you’d cover the iris with Viscoat in the angle all around the patient’s eye. Second, he says, you’d bridge the center of the anterior chamber with Healon5.
The third step is for you to stretch the pupil, which will not flop when covered and tamponaded with an OVD. Fourth, under the Healon5 layer, you’d inject a layer of xylocaine-phenylephrine solution to enhance pupil dilation. Finally, he explains that you’d perform phacoemulsification with a flow rate of 15 to 20 cc per minute, enabling the OVD to remain stationary throughout the procedure. “Taking this approach is simple and much easier than using rings,” argues Dr. Arshinoff.
Applying the Pressure
The final concept Dr. Arsinoff emphasizes involves the three levels of pressure when using OVDs:
• High pressure. Many surgeons prefer to work in high-pressure environments, using phaco machines that raise IOP to 60 or 80 mm Hg. “Higher pressure can be achieved with Healon5, Healon GV or with the use of different OVDs during SST,” he says.
• Medium pressure. Surgeons who use only dispersive OVDs primarily work in a medium-pressure environment. “Adaptations must be made to achieve some results in a lower-pressure environment,” says Dr. Arshinoff. “For example, avoiding the Argentinean Flag sign in a hypermature cataract is much easier with visco-adaptives, which can pressurize the anterior chamber to make the center of the anterior capsule concave for capsulorhexis.5 Reducing pressure makes this much more difficult.”
• Low pressure. Relying on low pressure, the opposite of what you would do when using visco-adaptives, is a typical approach in developing countries, where the cost of devices is a barrier. “Surgeons in those countries have devised ingenious techniques to manage cases in more difficult, low-pressure surgical environments,” Dr. Arshinoff says.
Why to Choose Wisely
What all of this means is that you can perform all of the steps that these experts have described by using randomly selected OVDs or no OVDs at all.
“But you’ll end up doing so with difficulty and, frequently, suboptimal results, compared to using OVDs that are optimally designed for what you’re trying to achieve,” says Dr. Arshinoff. “Each step of surgery is much easier when using the appropriate OVD. No OVD is ideal for everything.”
Dr. Arshinoff has Dr. consulted with every major manufacturer of OVDs. Dr. Miller is a consultant for J&J Vision and Alcon. Dr. Colvard reports no relationships with companies that make products related to this content.
1. Arshinoff SA. Using BSS with visco-adaptives in the ultimate soft-shell technique. J Cataract Refract Surg 2002;28:1509-14.
2. Arshinoff Steve A: Dispersive-cohesive viscoelastic soft shell technique. J. Cataract Refract Surg 1999;25; 2:167-173.
3. Arshinoff SA, Norman R. Tri-Soft Shell Technique. J Cataract Refract Surg 2013; 39:1196-1203.
4. Arshinoff SA. FLACS OVD Press to prevent radial anterior capsular tears. Can J Ophthalmol 2020;55:461-463. https://doi.org/10.1016/j.jcjo.2020.05.016.
5. Arshinoff Steve A. OVDs for intumescent cataracts: Pressure equalized cataract surgery. Letter. J Cataract Refract Surg 2015;41:1549-1550.
6. Arshinoff SA. Modified SST–USST for tamsulosin-associated intraocular floppy-iris syndrome. J Cataract Refract Surg 2006;32:559–561.