First described by Albrecht von Graefe in 1859,1 retinal artery occlusions are a well-known cause of significant visual morbidity. As suggested by Graefe’s initial work, patients with the condition may harbor serious underlying pathology, which may be life-threatening. Over the past 150 years, the etiologies, pathophysiology, clinical features, and natural history of RAOs have been extensively investigated.
More recently, to help RAO patients mitigate vision loss, prevent secondary cerebral stroke, and improve their quality of life, there has been a paradigm shift in the way health-care teams think about acute painless vision loss. When it comes to retinal artery occlusions, “time is retina,” much like “time is brain” for ischemic stroke.
In this article, we discuss the most recent guidelines regarding the workup and evaluation of patients with suspected RAOs and review the current evidence for proposed therapeutic interventions.
Pathogenesis
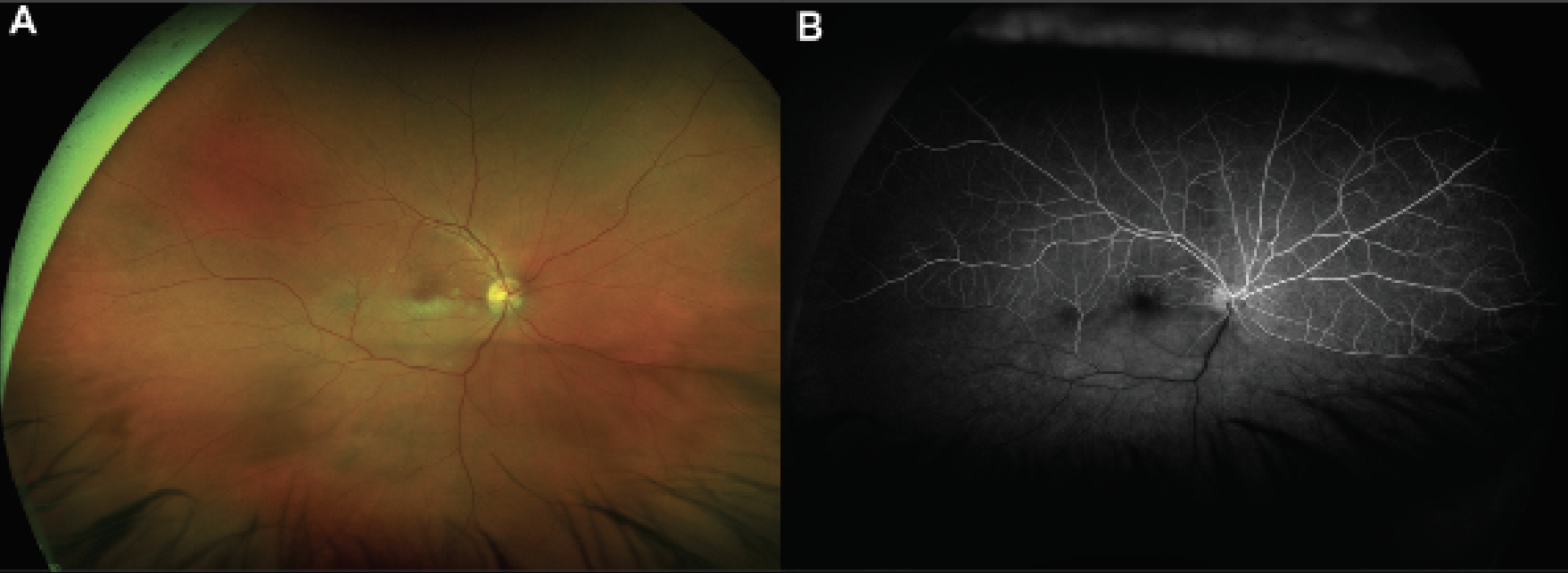
RAOs occur due to partial or complete cessation of blood flow through the central or branch retinal arteries. Once the initial vascular event has occurred, the compromised blood supply to the inner retinal layers leads to near immediate ischemia. Cytotoxic edema develops with resultant retinal whitening on fundoscopic examination (Figure 1A). Subsequently, a period of inflammation occurs in response to the damage. Thereafter, retinal atrophy and thinning develop around six weeks from the initial occlusion.
 |
|
Figure 1. Multimodal imaging of an acute branched retinal artery occlusion. A) Color fundus photograph of a patient with decreased vision that started 24 hours prior to presentation who was found to have inferior retinal whitening along the macula with associated emboli that was consistent with a branched retinal artery occlusion. B) Fluorescein angiogram at 30 seconds of the same patient highlights delayed perfusion of the inferior retina. |
In clinical practice, a major question arises as to when irreversible damage occurs. In the rhesus monkey, irreversible damage to the retina begins around 105 minutes2 after the inciting vaso-occlusive event, with massive irreversible retinal damage by 240 minutes.3 Of note, this experimental model involved placing a microclamp on the central retinal artery; the fidelity of this complete clamping model to the pathophysiology of real-world CRAs is unknown. More recent work proposes that complete occlusion of the CRA may result in retinal infarction in 12 to 15 minutes;4 however, cautious interpretation of this timeline is warranted as the claim is extrapolated indirectly from the brain-ischemia literature.5,6 Moreover, the real-world frequency of complete vessel occlusion as compared to partial vessel occlusion is unknown. Persistent retinal arterial perfusion beyond occlusions on fluorescein angiography and retinal vessel boxcarring further suggests partial vessel occlusion in at least some RAOs.
 |
Etiology
Retinal artery occlusions may occur due to a diverse array of etiologies. The most common causes of RAOs are summarized in Table 1. The vast majority of RAOs are embolic in nature (95 percent), with only 5 percent representing an arteritic etiology. Embolic RAOs may be differentiated based on exam features. Cholesterol emboli are the most common type and appear yellow, whereas platelet fibrin emboli appear gray, and calcium emboli appear white. One study found that among RAO emboli, 74 percent were cholesterol, 15 percent were platelet-fibrin and 11 percent were calcific.9
Less common types of emboli include tumor (e.g., atrial myxoma),10,11 leuko-embolus (e.g., pancreatitis),12 fat,13 air14 and septic15 emboli. In one prospective study of cardiovascular risk factors for CRAOs, 20 percent of patients with CRAO were found to have atrial fibrillation.16 Emboli causing CRAO may occur spontaneously or may be provoked in the setting of a recent intravascular procedure, such as cardiac catheterization.17 Injected cosmetic facial filler may embolize causing CRAO.18–20
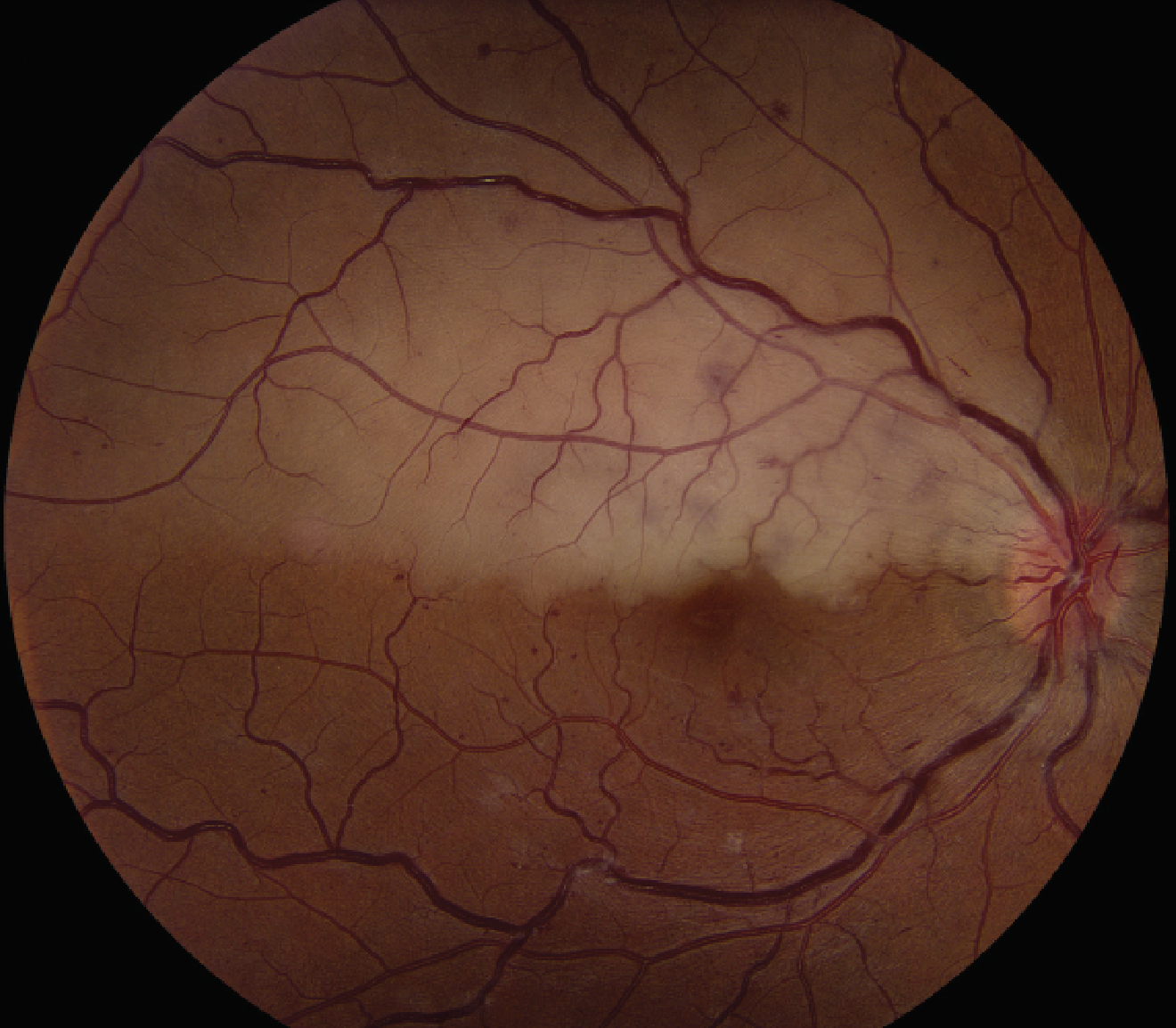
There are also multiple non-embolic etiologies of RAOs. Atherosclerosis of the central retinal artery may result in a RAO. Ocular trauma can lead to CRAO,21 with proposed mechanisms of insult including endothelial damage22 as well as direct vessel compression from optic nerve edema.23 Coagulopathic conditions, such as antiphospholipid syndrome,24 homocystinuria,25 protein S deficiency26 and nephrotic syndrome,27 may cause CRAO. Patients presenting with central retinal vein occlusion may infrequently have a concomitant RAO (Figure 2).28
A variety of inflammatory conditions have been linked to RAOs. These include giant cell arteritis (GCA),29 systemic lupus erythematosus,30 polyarteritis nodosa,31,32 granulomatosis with polyangiitis33,34 eosinophilic granulomatosis with polyangiitis,35,36 Behçet disease,37,38 Takayasu arteritis39 and fibromuscular dysplasia.40,41 Susac syndrome is another inflammatory condition that may cause RAO.42 The condition is characterized by multiple infarctions of the retina, cochlea and central nervous system; as such, patients may present with a triad of vision loss, sensorineural hearing loss and encephalopathy. Recurrent RAOs in the same patient should raise suspicion for the condition.43,44
 |
|
Figure 2. Color fundus photograph of a patient presenting with a combined retinal artery occlusion and central retinal vein occlusion. Fundus examination demonstrates increased vascular tortuosity, scattered intraretinal hemorrhages and retinal whitening along the superior macula. |
Rarely, infectious conditions have been implicated in RAOs such as SARS-COV-2 (COVID-19),45–49 Plasmodium falciparum (malaria),50 Rhizopus oryzae (mucormycosis)51 and Mycobacterium tuberculosis,52 among others.
RAO have also been reported after a number of ocular procedures and surgeries including retrobulbar anesthesia,53,54 intravitreal anti-vascular endothelial growth factor injection,55 cataract surgery with anterior vitrectomy,56 cataract surgery with sub-Tenon’s anesthesia57 and Descemet’s membrane detachment repair with pneumatic descematopexy,58 among others. Intraoperative factors that may increase risk for procedure-related RAO include IOP fluctuation as well as the use of adrenaline-containing anesthetic agents.59 Non-ophthalmic surgeries with face-down positioning may precipitate RAOs via prolonged extrinsic compression on the eye, as has been reported in association with spinal surgery.60–62
Malignancy has also been associated with RAOs; neoplastic infiltration of the optic nerve with CRAO has been described in patients with leukemia63–65 and lymphoma.66 CRAO has also been described in patients with solid malignancy, such as one patient with metastatic breast cancer involving the eye leading to combined CRAO and CRVO.67
Symptoms
Patients with RAOs may report symptoms of monocular vision loss that is sudden (seconds) and painless. Vision loss may include a total visual field defect or a hemifield defect in the case of a branch occlusions. There may have been transient episodes of vision loss (amaurosis fugax) that preceded the permanent vision loss.68–70 The duration of vision loss should be reported and patients should be queried regarding recent events such as trauma, surgery/procedure or recent illness.
In patients over the age of 50, symptoms of GCA should be assessed and documented (malaise, fatigue, myalgia, fever, temporal and scalp tenderness, jaw claudication, headache and diplopia). Preceding trauma with neck or facial pain should raise suspicion for carotid artery dissection71,72 and prompt further investigation.
Patient Evaluation
In all cases of suspected RAO, a thorough medical history should be conducted. Clinicians should review the patient’s medications as well as medical, ocular and surgical histories. Particular care should be given to systemic cardiovascular disease, hematologic conditions, malignancy and rheumatologic issues.
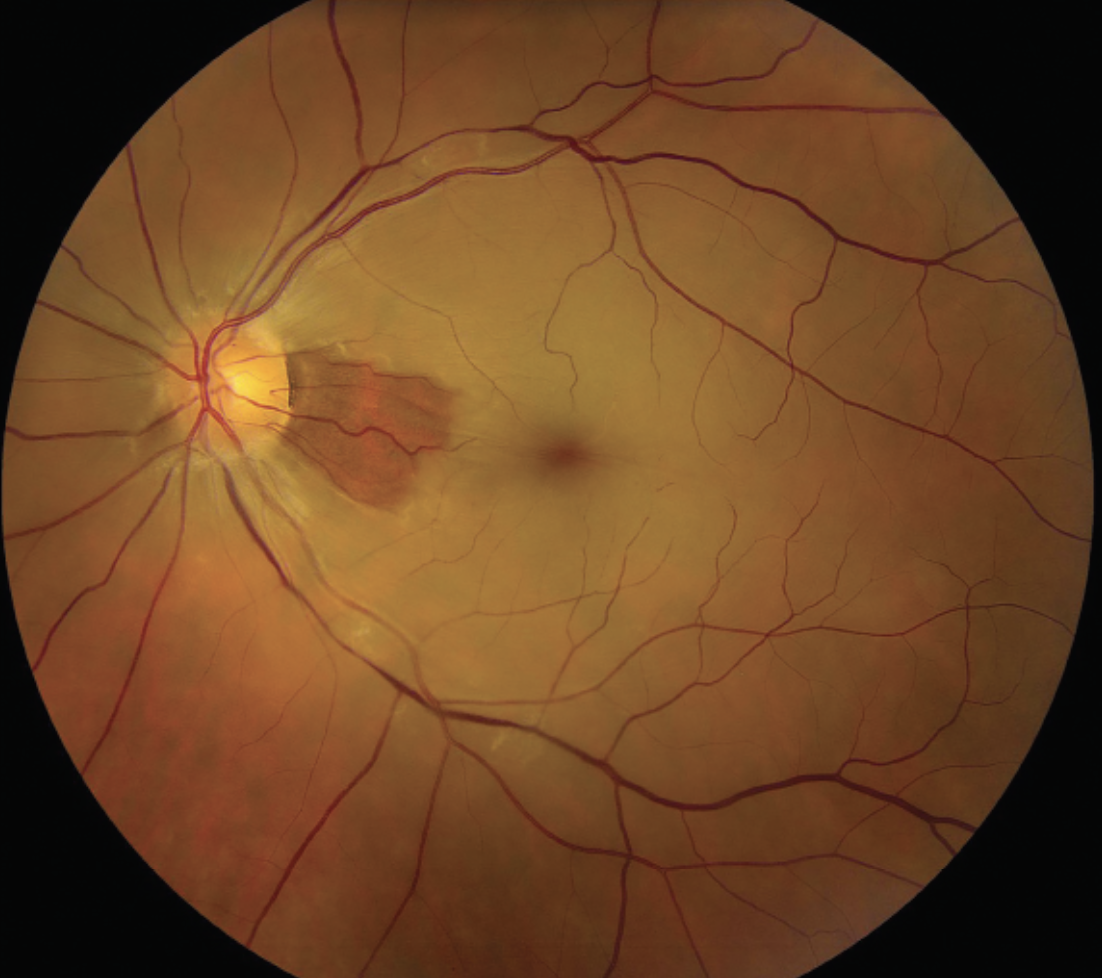 |
| Figure 3. Color fundus photograph of a patient with a central retinal artery occlusion with cilioretinal sparing. There’s a cherry red spot with associated retinal whitening involving the entire macula except for the area of the cilioretinal retinal artery along the nasal macula. |
The degree of vision loss in RAO is often related to the location of the occlusion in the vascular tree. Among patients with BRAO, 74 percent of patients had an initial visual acuity of 20/40 or better; whereas in those diagnosed with a CRAO, 74 percent had an initial visual acuity of count fingers or worse.73 In contrast, patients with occlusion of the ophthalmic artery often have a presenting visual acuity of light perception only or worse. An isolated cilioretinal artery occlusion should prompt consideration for GCA workup.74
Fundoscopic evaluation of RAO will reveal a cascade of pathologic changes to the retina. As the retinal nerve fiber layer becomes edematous due to ischemia, retinal whitening will occur in the distribution of affected perfusion. In cases of CRAO, a cherry-red spot appears in the fovea due to the histologic absence of the retinal nerve fiber layer in this region (Figure 3). The degree of retinal edema may serve as a proxy for the degree of retinal ischemia.75,76 In four to six weeks following the occlusion, the retinal whitening dissipates. During this period, the retinal vessels become attenuated; other late stage retinal findings include macular retinal pigment epithelial changes and cilioretinal collaterals.77
In most cases of RAO, the optic disc initially appears normal or unaffected by the condition. However, optic disc edema with RAO may signal involvement of the posterior ciliary arteries or an occlusion of the ophthalmic artery. In this scenario, a vasculitis involving the posterior ciliary arteries should be considered, including GCA. In one prospective study of patients with biopsy-proven GCA, 14 percent of patients had concurrent RAO and 81 percent had anterior ischemic optic neuropathy.29 In the late stages of RAOs, the optic nerves may develop pallor.77
Imaging
Multimodal ocular imaging may assist in confirmation of an RAO diagnosis but may not be required as the diagnosis can be made clinically. Importantly, ancillary imaging testing shouldn’t delay transfer to a stroke center, as suspicion for RAO is a medical emergency requiring stroke evaluation.78
Optical coherence tomography is often the quickest modality to confirm the diagnosis. In the acute period after occlusion, the inner retina has increased thickness and appears hyperreflective from the ischemic process, whereas the outer retina appears hyporeflective (Figure 4A). OCT features may disclose prognostic information to the clinician as well: Increased central macular thickness on OCT at baseline may be related to worse final vision and suggested an increased degree of ischemia in one study.79 Within four to six weeks from the initial event, the inner retina appears thin and atrophic, while the outer retina laminations and RPE/choriocapillaris remain unchanged (Figure 4B).80
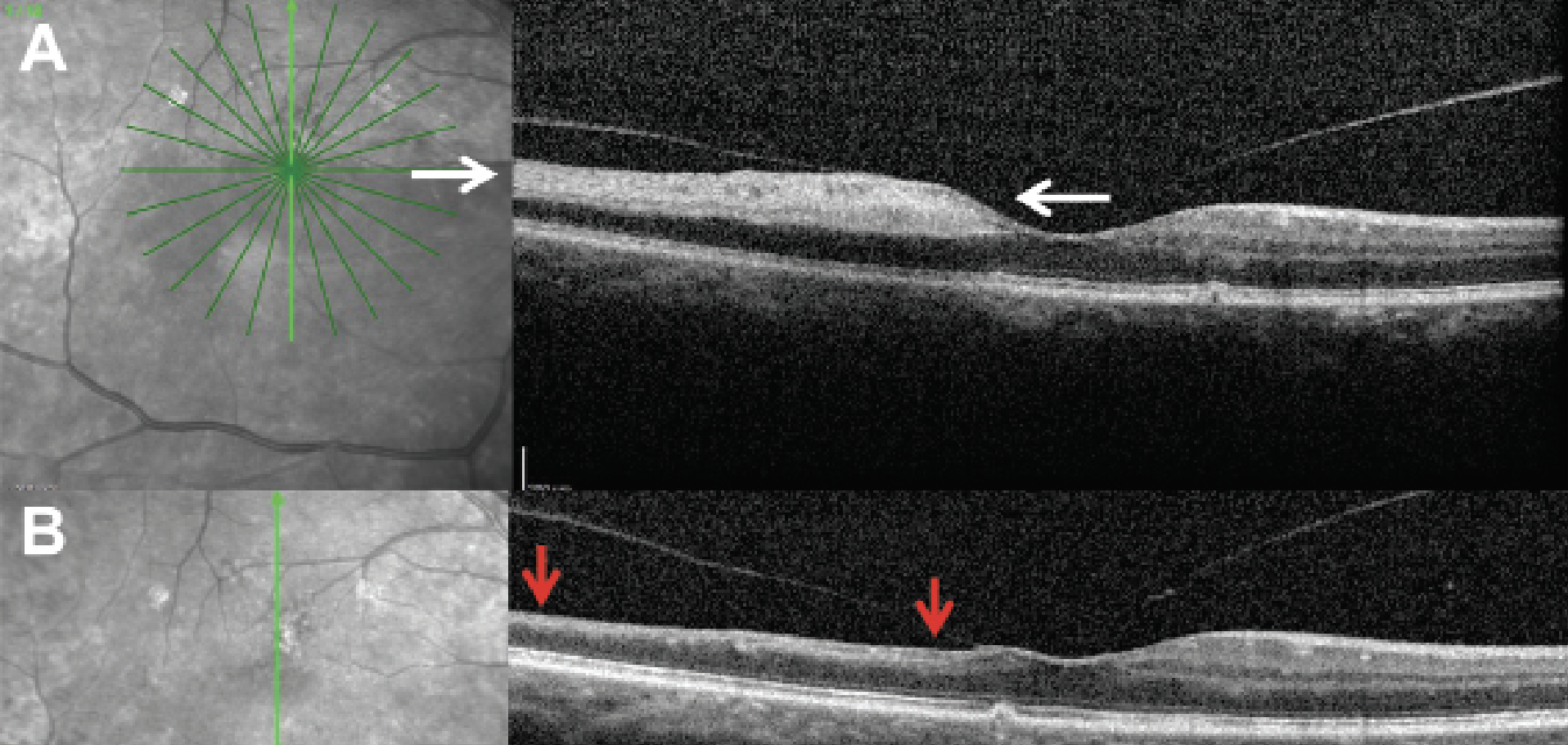 |
|
Figure 4. Optical coherence tomography of a branched retinal artery occlusion in the acute and chronic phase. A) OCT of a branched retinal artery occlusion within 24 hours of symptom onset. The OCT demonstrates increased thickness and appears hyperreflective (white arrows) while the corresponding areas of the outer retina appear relatively hyporeflective. B) Three months after initial presentation, OCT of the same patient shows signs of inner retinal atrophy in the areas that were previously hyperreflective (red arrows). |
Fluorescein angiography may serve as a useful diagnostic tool when the etiology of the RAO is unclear (Figure 1B). Choroidal perfusion is typically normal in FA of RAO; however, perfusion abnormalities of the choroid in suspected RAO suggests involvement of the posterior ciliary arteries (such as in GCA) or a more proximal lesion such as an ophthalmic artery occlusion. Choroidal perfusion defects on FA in the absence of emboli on examination should warrant a GCA evaluation as part of the stroke workup.
Systemic Evaluation
Upon initial diagnosis of a retinal artery occlusion, transfer to a stroke center for evaluation is a crucial step, as risk of strokes is significantly increased, especially within the first one to four weeks.81,82 In one study, 23 percent of patients with CRAO showed acute brain infarcts on MRI within an average of 24 hours from symptom onset. Among those patients with acute brain infarcts, 89 percent reported no additional neurologic symptoms.83 Another study found that 71 percent of non-arteritic CRAO had internal carotid plaques and that 52 percent of non-arteritic CRAO had abnormal echocardiogram with a suspected embolic source.84 With regard to mortality risk, a population-based study in South Korea found that patients with RAO had a 7.33 higher risk (standardized mortality ratio) of all-cause mortality as compared to that of age-matched controls in the general population.85
A stroke workup often involves a neurology consultation with a prompt systemic evaluation for carotid occlusive and thromboembolic disease. Urgent imaging is often acquired including MRI brain, echocardiography, electrocardiogram and/or carotid artery dopplers. Systemic laboratory evaluation may include fasting blood sugar, hemoglobin A1C, complete blood count with differential and lipid profiles. In younger patients (under 50 years old), a workup for vasculitis or hypercoagulability may be indicated. In patients over 50, one must additionally suspect GCA; urgent corticosteroid therapy should be considered when GCA is diagnosed or is very likely to preserve vision in the affected eye and contralateral eye.
Despite the potential for systemic comorbidities upon initial RAO diagnosis, studies have indicated that urgent transfer to a stroke center upon RAO diagnosis may be limited by practice patterns, health-care system resources and patient medical literacy, among other factors.86,87 Part of these discrepancies may arise when patients present with symptomatic vs asymptomatic RAOs. Acute, symptomatic RAOs should prompt an immediate referral to the nearest stroke referral center for prompt assessment for consideration of an acute intervention. The precise timing of evaluation for patients with an asymptomatic but newly diagnosed RAO is unclear, though these patients still warrant a timely referral. In our practice, all patients with asymptomatic BRAOs or CRAOs are routinely referred for an expedited stroke workup.
To minimize delays in care, treating ophthalmologists are encouraged to establish relationships with local stroke centers in their respective areas of practice. Resources should be directed towards alerting community practitioners in neurology, primary care and emergency medicine that acute, painless monocular visual loss can be a stroke analogue that needs to be assessed at a stroke center. Education regarding the potential impact that this diagnosis may have on their patients’ future risks of stroke, myocardial infarction and death is also essential.
Treatment
Despite the significant interest, a safe and effective treatment remains elusive for RAOs. The sensitivity of retinal tissue to ischemia makes the therapeutic window for intervention in RAO small, likely between 90 to 240 minutes.2,3 If an effective treatment were to become available, this narrow window would likely require significant public health education and health-care resource allocation to translate to a real-world reduction in visual morbidity. Current treatment guidelines focus on harm reduction for further sequelae of the underlying etiology of the RAO.
Several interventions have been trialed, though most have yielded ineffective or mixed results; moreover, some of these interventions confer considerable risk for serious side effects. A reduction in intraocular pressure to decrease resistance of retinal arterioles has been proposed as one treatment mechanism. Such a mechanism can be achieved via IV acetazolamide or mannitol, topical intraocular pressure drops, or anterior chamber paracentesis. Digital ocular massage leads to fluctuation in intraocular pressure to theoretically propagate the clot distally. Other proposed mechanisms of treatment include vasodilation to increase blood oxygen content using pentoxifylline, inhalation of carbogen, or sublingual isosorbide dinitrate.88 Hyperbaric oxygen therapy increases blood oxygen tension, which has been proposed as a treatment option.89
Despite these potential mechanisms, there is no level I evidence to support any of these wide array of therapies. Furthermore, in one meta-analysis, conservative treatment measures (ocular massage, anterior chamber paracentesis and/or hemodilution) had a worse visual recovery rate than that of the natural history group; the number needed to harm in the conservative treatment group was 10.90
Both intra-arterial and intravenous thrombolytics have been investigated in RAO management; however, there is strong controversy regarding their use. For intra-arterial thrombolytics, this procedure is performed by a neuro-radiologist, complicating the logistics of care and prolonging the time between symptom onset and intervention. Via superselective microcatheterization, tPA is introduced into the ostium of the ophthalmic artery. Microvascular access mitigates systemic complications, but increases risk of catheter-induced spasm, arterial dissection and plaque dislodgement.91
The EAGLE trial (European Assessment Group for Lysis in the Eye) comparing intra-arterial lysis to conservative treatment was terminated prematurely due to a profound safety signal: 37.1 percent vs 3.4 percent risk of adverse events, respectively. Moreover, visual outcomes were similar between the two groups.92 Intravenous thrombolytics also risk adverse events such as intracranial hemorrhage or death with no or minimal visual acuity gains reported.93,94
For non-arteritic RAO, there is no current treatment that is proven to yield superior outcomes when compared to that of the natural disease course. As has been highlighted before,70 fibrinolysis doesn’t significantly dissolve calcific or cholesterol emboli; as such the therapy only has a plausible mechanism of action for a minority of cases (platelet-fibrin emboli).
After the initial disease course, patients should still be regularly followed by an ophthalmologist to evaluate for ocular neovascularization. When anterior segment neovascularization develops, panretinal photocoagulation as well as off-label use of intravitreal anti-VEGF agents may be warranted to minimize the risk of neovascular glaucoma.
In conclusion, retinal artery occlusions are an ophthalmic emergency requiring detailed systemic workup. Although the visual prognosis in patients with RAO may be guarded, a prompt systemic workup is crucial as RAOs may be a harbinger of other systemic disease. Given the limited treatment armamentarium, investigation into novel therapeutic interventions for RAOs may confer significant benefit to future patients. Much work is left to be done for patients with this high-morbidity condition.
Dr. Wibbelsman (PGY-2) and Dr. Massenzio (PGY-4) are resident physicians at Wills Eye Hospital in Philadelphia. Dr. Patel is a vitreoretinal surgeon at Wills Eye Hospital/Mid Atlantic Retina.
1. Graefe A. Ueber Embolie der Arteria centralis retinae als Ursache plötzlicher Erblindung. Arch für Ophthalmol 1859;5:136-157.
2. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology 1980;87:1:75-78.
3. Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Exp Eye Res 2004;78:3:723-736.
4. Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion—Rethinking retinal survival time. BMC Ophthalmol 2018;18:1.
5. Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981;12:6:723-725.
6. Raichle ME. The pathophysiology of brain ischemia. Ann Neurol 1983;13:1:2-10.
7. Jumper JM, Horton JC. Central retinal artery occlusion after manipulation of the neck by a chiropractor. Am J Ophthalmol 1996;121:3:321-322.
8. Greven MA, Moshfeghi DM. Strangulation-induced central retinal artery occlusion: Case report and review of the literature. Retin Cases Brief Rep 2017;11:3:258-260.
9. Arruga J, Sanders MD. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology 1982;89:12:1336-1347.
10. Yi SY, Han MJ, Kong YH, Joo CU, Kim SJ. Acute blindness as a presenting sign of left atrial myxoma in a pediatric patient: A case report and literature review. Medicine (Baltimore) 2019;98:38.
11. Balasubramaniam S, Sreenivasan J, Dhawan A, Agarwal A. A case of central retinal artery occlusion due to atrial myxoma with excellent visual recovery. Indian J Ophthalmol 2022;70:7:2698.
12. Timoney PJ, Pate JC, Pearson PA, Crandall J. Bilateral central retinal artery occlusion in a patient with acute pancreatitis. Retin Cases Brief Rep 2009;3:3:308-309.
13. Kim KE, Ahn SJ, Woo SJ, Kim N, Hwang JM. Central retinal artery occlusion caused by fat embolism following endoscopic sinus surgery. J Neuro-Ophthalmology 2013;33:2:149-150.
14. Lee J, Chin JH, Koh WU, Ro YJ, Yang HS. Unilateral postoperative visual loss in a patient undergoing hip arthroscopy in the supine position: A case report. Korean J Anesthesiol 2016;69:2:197-199.
15. Mohamed M, Scholle T. Bilateral central retinal artery occlusion in infective endocarditis. J Vitreoretin Dis 2021;5:3:261.
16. Callizo J, Feltgen N, Pantenburg S, et al. Cardiovascular risk factors in central retinal artery occlusion: Results of a prospective and standardized medical examination. Ophthalmology 2015;122:9:1881-1888.
17. Nakata A, Sekiguchi Y, Hirota S, Yamashita Y, Takazakura E. Central retinal artery occlusion following cardiac catheterization. Jpn Heart J 2002;43:2:187-192.
18. Wang I, Lin HJ, Tsai YY, et al. Multiple branch retinal artery occlusions following the new facial cosmetic filler (Poly-D, L-lactic Acid) injection a case report. BMC Ophthalmol 2023;23:1:1-6.
19. Davidova P, Müller M, Wenner Y, König C, Kenikstul N, Kohnen T. Ophthalmic artery occlusion after glabellar hyaluronic acid filler injection. Am J Ophthalmol Case Reports 2022;26:101407.
20. Si M, Wang H. Retinal artery occlusion after facial filler injection in a patient with patent foramen ovale: A case report and literature review. J Int Med Res 2023;51:9:1.
21. Karande S, Khalsa A, Kelgaonkar A. Central retinal artery occlusion: A manifestation of blunt trauma. BMJ Case Rep 2020;13:8:235632.
22. Dalma-Weiszhausz J, Meza-de Regil A, Martínez-Jardón S, Oliver-Fernández K. Retinal vascular occlusion following ocular contusion. Graefes Arch Clin Exp Ophthalmol 2005;243:5:406-409.
23. Sakamoto S ichi, Makino S, Kawashima H. Traumatic optic neuropathy and central retinal artery occlusion following blunt trauma to the eyebrow. J Gen Fam Med 2017;18:6:456-457.
24. Joshi U, Afroz S, Ranka S, Mba B. Bilateral central retinal artery occlusion from catastrophic antiphospholipid syndrome. BMJ Case Rep. Nov 12, 2018.
25. Zahid S, Iqbal M, Ubaid A, Khan F. Homocystinuria in a 14-year old girl manifesting as central retinal artery occlusion: A case report. J Pak Med Assoc 2020;70:7:1263-1265.
26. Golub BM, Sibony PA, Coller BS. Protein S deficiency associated with central retinal artery occlusion. Arch Ophthalmol 1990;108:7:918-918.
27. Sinha S, Rau ATK, Kumar R V., Jayadev C, Vinekar A. Bilateral combined central retinal artery and vein occlusion in a 3-year-old child with nephrotic syndrome. Indian J Ophthalmol 2018;66:10:1498-1501.
28. Brown GC, Duker JS, Lehman R, Eagle RC. Combined central retinal artery-central vein obstruction. Int Ophthalmol 1993;17:1:9-17.
29. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol 1998;125:4:509-520.
30. Lim Y, Afkhamnejad ER, Kavoussi S. Unilateral CRAO as the presenting clinical sign of systemic lupus erythematosus. Am J Ophthalmol Case Reports 2023;29:101810.
31. Hsu CT, Kerrison JB, Miller NR, Goldberg MF. Choroidal infarction, anterior ischemic optic neuropathy, and central retinal artery occlusion from polyarteritis nodosa. Retina 2001;21:4:348-351.
32. Emad Y, Basaffar S, Ragab Y, Zeinhom F, Gheita T. A case of polyarteritis nodosa complicated by left central retinal artery occlusion, ischemic optic neuropathy, and retinal vasculitis. Clin Rheumatol 2007;26:5:814-816.
33. Shim KY, Sohn S-I, Kim YC. Central retinal arterial occlusion in granulomatosis with polyangiitis. Korean J Ophthalmol 2018;32:6:519.
34. Mirza S, Ram ARR, Bowling BS, Nessim M. Central retinal artery occlusion and bilateral choroidal infarcts in Wegener’s granulomatosis. Eye (Lond) 1999;13:Pt 3a(3):374-376.
35. Nishiyama H, Tajiri T, Yamabe T, et al. Eosinophilic granulomatosis with polyangiitis presenting with central retinal artery occlusion during treatment with anti-interleukin-5 receptor monoclonal antibody. Intern Med 2021;60:22:3631.
36. Park J, Huh U, Choi HY, et al. Central retinal artery occlusion in eosinophilic granulomatosis with polyangiitis (Churg-Strauss Syndrome): The first case report in South Korea. Int J Ophthalmol 2021;14:6:948.
37. Özdal PÇ, Ortaç S, Taskintuna I, Teke MY, Firat E. Central retinal artery occlusion associated with ocular Behçet’s disease. Eur J Ophthalmol 2002;12:4:328-330.
38. Esen E, Sizmaz S, Sariyeva A, Demircan N. Bilateral central retinal artery occlusion in Behçet disease. Ocul Immunol Inflamm 2015;23:5:416-419.
39. Guclu H, Gurlu VP, Ozal SA, Guclu O. Central retinal artery occlusion in Takayasu’s arteritis as the first presentation of the disease. Case Rep Ophthalmol Med 2016;1-3.
40. Mehkri Y, Poe J, Murshid M, et al. Retinal artery occlusion in fibromuscular dysplasia: A case report. Cureus 2022;14:11.
41. Altun A, Altun G, Olcaysu OO, Kurna SA, Aki SF. Central retinal artery occlusion in association with fibromuscular dysplasia. Clin Ophthalmol 2013;7:2253-2255.
42. Buelens T, Herode L, Nubourgh I, Caspers L, Willermain F, Postelmans L. Central retinal artery occlusion and Susac syndrome: A case report. Retin Cases Brief Rep 2014;8:3:187-192.
43. Dörr J, Krautwald S, Paul F, et al. Characteristics of Susac syndrome: A review of all reported cases. Nat Rev Neurol 2013;9:6:307-316.
44. Haefliger E, Marbet GA, Hotz G. Multiple recurrent occlusions of the retinal and cerebral arteries in a 20-year-old woman. Klin Monbl Augenheilkd 1982;181:2:103-107.
45. Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases 2020;21.
46. Bapaye MM, Nair AG, Bapaye CM, Bapaye MM, Shukla JJ. Simultaneous bilateral central retinal artery occlusion following COVID-19 infection. Ocul Immunol Inflamm 2021;29:4:671-674.
47. Uzun A, Keles Sahin A, Bektas O. A unique case of branch retinal artery occlusion associated with a relatively mild coronavirus disease 2019. Ocul Immunol Inflamm 2021;29:4:715-718.
48. Montesel A, Bucolo C, Mouvet V, Moret E, Eandi CM. Case report: Central retinal artery occlusion in a COVID-19 patient. Front Pharmacol 2020;11.
49. Ucar F, Cetinkaya S. Central retinal artery occlusion in a patient who contracted COVID-19 and review of similar cases. BMJ Case Rep 2021;14:7.
50. Choudhury H, Panchal B, Doshi S, Pathengay A. Case report: Occlusion of the central retinal artery in Plasmodium falciparum malaria. BMJ Case Rep 2019;12:7.
51. Bawankar P, Lahane S, Pathak P, Gonde P, Singh A. Central retinal artery occlusion as the presenting manifestation of invasive rhino-orbital-cerebral mucormycosis. Taiwan J Ophthalmol 2020;10:1:62.
52. Ooi YL, Tai LY, Subrayan V, Tajunisah I. Combined optic neuropathy and central retinal artery occlusion in presumed ocular tuberculosis without detectable systemic infection. Ocul Immunol Inflamm 2011;19:5:370-372.
53. Vasavada D, Baskaran P, Ramakrishnan S. Retinal vascular occlusion secondary to retrobulbar injection: Case report and literature review. Middle East Afr J Ophthalmol 2017;24:1:57-60.
54. Jung EH, Park KH, Woo SJ. Iatrogenic central retinal artery occlusion following retrobulbar anesthesia for intraocular surgery. Korean J Ophthalmol 2015;29:4:233-240.
55. Gao X, Borkar D, Obeid A, Hsu J, Ho AC, Garg SJ. Incidence of retinal artery occlusion following intravitreal antivascular endothelial growth factor injections. Acta Ophthalmol 2019;97:6:e938-e939.
56. Sen A, Mitra A, Tripathi S, Sharma M, Shenoy P. A cluster of central retinal artery occlusions following cataract surgery. Indian J Ophthalmol 2019;67:5:630-633.
57. Dragnev D, Barr D, Kulshrestha M, Shanmugalingam S. A case of branch retinal artery occlusion following uneventful phacoemulsification. Case Rep Ophthalmol 2013;4:1:27.
58. Meena AK, Ghodke BR, Parmar GS. Central retinal artery occlusion after Descemet membrane reposition by intracameral air: A case report. https://doi.org/101177/1120672119870740. 2019;31:2:NP77-NP80.
59. Deshmukh R, Narula R. Commentary: Central retinal arterial occlusions after phacoemulsification: Our perspective. Indian J Ophthalmol 2019;67:5:633-634.
60. Bekar A, Türeyen K, Aksoy K. Unilateral blindness due to patient positioning during cervical syringomyelia surgery: Unilateral blindness after prone position. J Neurosurg Anesthesiol 1996;8:3:227-229.
61. Ward WT, Grossman W. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976) 1993;18:9:1226-1228.
62. Sys J, Michielsen J, Mertens E, Verstreken J, Tassignon MJ. Central retinal artery occlusion after spinal surgery. Eur Spine J 1996;5:1:74-75.
63. Khair D, Mehanna CJ, Ghannam AB, Kheir WJ. Bilateral retinal artery occlusions as the first manifestation of extramedullary central nervous system involvement in relapsed acute myeloid leukaemia. BMJ Case Rep 2021;14:4:239795.
64. Zhao C, Wei D, Shi X, Zhao M. Unilateral isolated optic nerve infiltration combined with central retinal artery occlusion in a patient with acute myeloid leukemia. Am J Ophthalmol Case Reports 2022;26:101493.
65. Iwami T, Nishida Y, Mukaisho M, Kani K, Narita T, Taga T. Central retinal artery occlusion associated with leukemic optic neuropathy. J Pediatr Ophthalmol Strabismus 2003;40:1:54-56.
66. Desouza PJ, Hooten CG, Lack CM, John VJ, Martin TJ. Bilateral central retinal artery occlusion associated with bilateral lymphoproliferative infiltrative optic neuropathy. Ocul Oncol Pathol 2017;3:3:229.
67. Khan M, Gonzalez LA, Papakostas TD. Optic nerve metastasis causing a combined central retinal artery and vein occlusion in a breast cancer patient. Ophthalmology Retina 2020;4:11:1058.
68. Terao R, Fujino R, Ahmed T. Risk factors and treatment strategy for retinal vascular occlusive diseases. J Clin Med 2022;11:21.
69. Vodopivec I, Cestari DM, Rizzo JF. Management of transient monocular vision loss and retinal artery occlusions. Semin Ophthalmol 2017;32:1:125-133.
70. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: Visual outcome. Am J Ophthalmol 2005;140:3:376.e1-376.e.
71. Mazzeo TJMM, Freire RCM, Filho LF, Machado CG, Gomes AMV. Central retinal artery occlusion secondary to presumed traumatic carotid artery dissection in a healthy child. Int J Retin Vitr 2022;8:1:56.
72. Hendricks BK, Ding D, Almefty RO, Albuquerque FC, Ducruet AF. Carotid Dissection (Chapter TBD). In: Park MS, Kalani MYS, DeHavenon A, McNally JS, eds. Carotid Artery Disease—Evaluation and Management. New York: Springer, 2020:155-172.
73. Hayreh SS. Ocular vascular occlusive disorders: Natural history of visual outcome. Prog Retin Eye Res 2014;0:1:1.
74. Rizzo C, Kilian R, Savastano MC, Fossataro C, Savastano A, Rizzo S. A case of cilioretinal artery occlusion: Diagnostic procedures. Am J Ophthalmol Case Reports 2023;32:101949.
75. Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2013;54:12:7746-7755.
76. Furashova O, Matthé E. Retinal changes in different grades of retinal artery occlusion: An optical coherence tomography study. Invest Ophthalmol Vis Sci 2017;58:12:5209-5216.
77. Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina 2007;27:3:276-289.
78. Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal and ophthalmic artery occlusions preferred practice pattern. Ophthalmology 2020;127:2:P259-P287.
79. Ahn SJ, Woo SJ, Park KH, Jung C, Hong JH, Han MK. Retinal and choroidal changes and visual outcome in central retinal artery occlusion: An optical coherence tomography study. Am J Ophthalmol 2015;159:4:667-676.e1.
80. Falkenberry SM, Ip MS, Blodi BA, Gunther JB. Optical coherence tomography findings in central retinal artery occlusion. Ophthalmic Surg Lasers Imaging 2006;37:6:502-505.
81. Park SJ, Choi NK, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology 2015;122:11:2336-2343.e2.
82. Golsari A, Bittersohl D, Cheng B, et al. Silent brain infarctions and leukoaraiosis in patients with retinal ischemia: A prospective single-center observational study. Stroke 2017;48:5:1392-1396.
83. Lauda F, Neugebauer H, Reiber L, Jüttler E. Acute silent brain infarction in monocular visual loss of ischemic origin. Cerebrovasc Dis 2015;40:3-4:151-156.
84. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: Associated systemic and ophthalmic abnormalities. Ophthalmology 2009;116:10:1928-1936.
85. Hwang DDJ, Lee KE, Kim Y, et al. Incidence of retinal artery occlusion and related mortality in Korea, 2005 to 2018. JAMA Netw Open 2023;6:3:e233068-e233068.
86. Atkins EJ, Bruce BB, Newman NJ, Biousse V. Translation of clinical studies to clinical practice: Survey on the treatment of central retinal artery occlusion. Am J Ophthalmol 2009;148:1:172-173.
87. Flowers AM, Chan W, Meyer BI, Bruce BB, Newman NJ, Biousse V. Referral patterns of central retinal artery occlusion to an academic center affiliated with a stroke center. J Neuroophthalmol 2021;41:4:480.
88. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol 2013;15:1:63.
89. Wu X, Chen S, Li S, et al. Oxygen therapy in patients with retinal artery occlusion: A meta-analysis. PLoS One 2018;13:8. doi:10.1371/JOURNAL.PONE.0202154
90. Dumitrascu OM, Shen JF, Kurli M, et al. Is intravenous thrombolysis safe and effective in central retinal artery occlusion? A critically appraised topic. Neurologist 2017;22:4:153-156.
91. MacGrory B, Schrag M, Biousse V, et al. Management of central retinal artery occlusion: A scientific statement from the American Heart Association. Stroke 2021;52:6:E282-E294.
92. Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: Local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010;117:7:1367-1375.e1.
93. Chen CS, Lee AW, Campbell B, et al. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion. Stroke 2011;42:8:2229-2234.
94. Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous fibrinolytic therapy in central retinal artery occlusion: A patient-level meta-analysis. JAMA Neurol 2015;72:10:1148-1154.



