Ab interno canaloplasty and gonioscopy-assisted transluminal trabeculotomy, or GATT, have been in use for several years. Each has a track record of effective intraocular pressure lowering in many patients, but they aim at different targets; GATT addresses resistance at the trabecular meshwork by unroofing it, while canaloplasty addresses resistance at the trabecular meshwork, inside Schlemm’s canal and in the collector channels through which aqueous exits the anterior chamber, by inflating Schlemm’s canal from the inside with viscoelastic.
Recently, we’ve been combining these procedures, with a modification to the canaloplasty procedure that helps reveal the condition of the post-trabecular aqueous outflow system. In this article we’d like to explain the rationale behind our modified combination procedure and describe how it’s performed, in the hope that this will inspire further investigation and advancement in this area.
Using Canaloplasty
Canaloplasty is a therapeutic procedure. Basically, it involves injecting viscoelastic into Schlemm’s canal; the OVD also works its way through the collector channels and aqueous veins. This procedure is thought to increase outflow through a number of mechanisms: 1) creating microfenestrations in the trabecular meshwork, thereby reducing resistance at this level; 2) releasing intracanalicular adhesions and clear-ing debris within collapsed portions of the post-trabecular outflow system; and 3) opening physiologic valves that regulate outflow.
 |
In the past, reduced outflow was thought to be the result of debris or atrophy blocking a simple, stationary filtering system, with the trabecular meshwork acting as the filter. Since then, Murray Johnstone, MD, has demonstrated that the outflow system involves multiple one-way valves that help to move fluid from Schlemm’s canal into the collector channels in a pulsatile manner, similar to the way lymphatic veins have been shown to work.1 (This comparison has been supported by work done at the University of Toronto by Yeni Yucel, MD.2) Injecting viscoelastic during canaloplasty may permanently force open those valves, thus decreasing the resistance to flow through the pathway. Therefore, it’s possible that this procedure is not just clearing away debris inside Schlemm’s canal, but is actually making the outflow system more efficient.
There are two approaches to performing canaloplasty: ab externo and ab interno. (We prefer the latter.) Ab externo was the method initially described. In this version, the surgeon creates a partial-thickness scleral flap allowing the insertion of a catheter into Schlemm’s canal. In the ab interno version, we enter the eye via a clear corneal incision and access Schlemm’s canal through a small goniotomy. (Both versions of the canaloplasty rely on the Ellex’s iTrack 250-µm microcatheter, which incorporates a fiber optic light that allows the surgeon to visualize the progression of the catheter’s tip during the 360-degree insertion.)
One significant advantage of ab interno canaloplasty is that we pre-serve the conjunctiva, which is important when managing glaucoma. As glaucoma surgeons, we’re always planning our next move in case the current procedure doesn’t work well enough. (Unfortunately, glaucoma patients tend to progress despite our best efforts.) Preserving conjunctiva and minimizing scarring increases the odds of success if we need to do a trabeculectomy or tube later on.
 |
Trypan Blue Venography
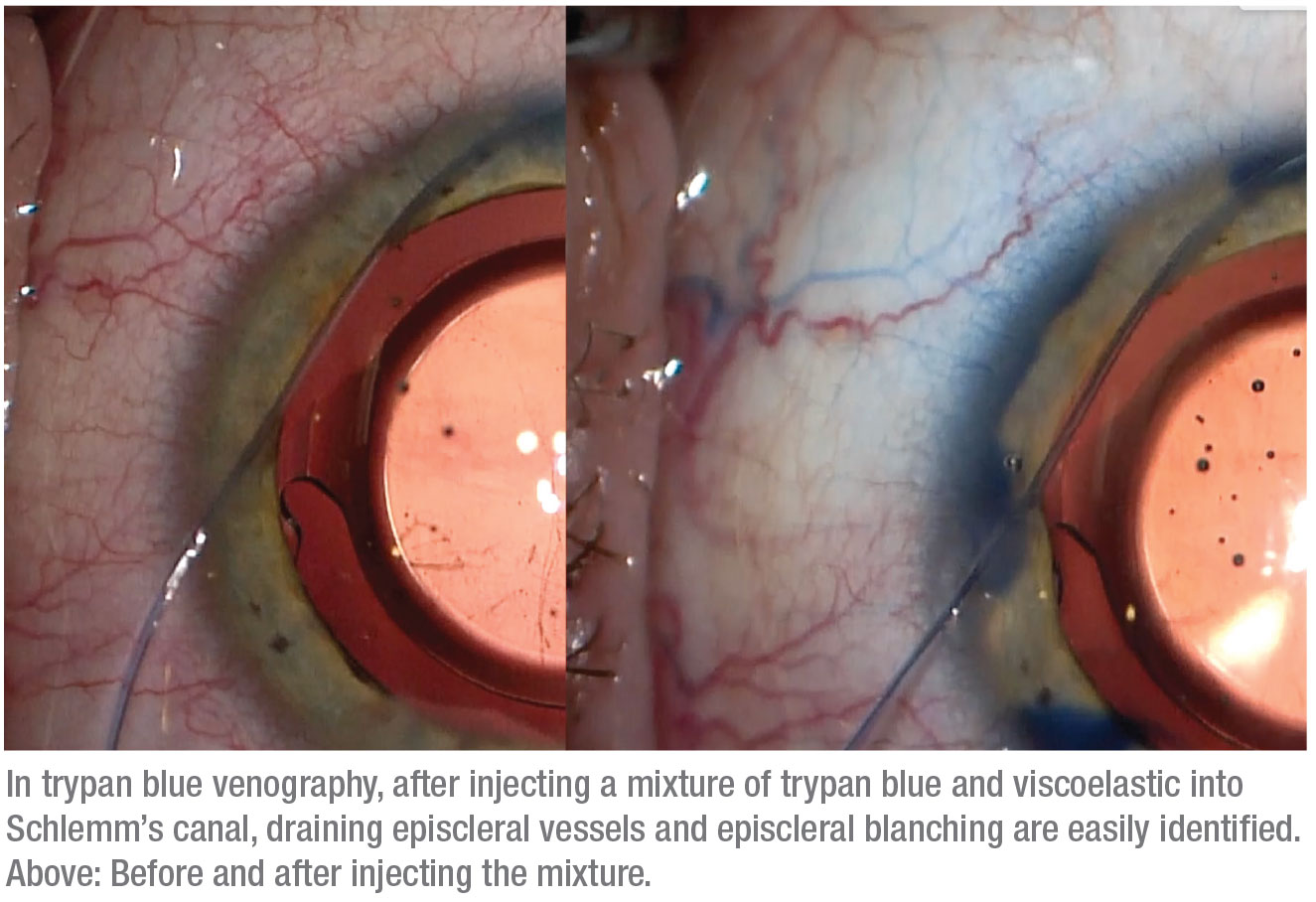
During canaloplasty we pressurize Schlemm’s canal with viscoelastic, and some of it is distributed through any functioning collector channels. We realized that this provides an opportunity to visualize the status of the post-trabecular outflow system; all that’s required is to make the viscoelastic easier to see. That’s easily accomplished by adding trypan blue to the viscoelastic before it’s injected into the canal. Seeing where the viscoelastic is exiting into the collector channels and outflow system (which may include lymphatics in addition to veins) can help us make treatment choices based on information we haven’t previously had, while also helping to increase our understanding of the outflow system in general.
 |
A related idea using balanced salt solution to look for what’s called the “fluid wave sign” has previously been described. In this method, when the anterior chamber is hyperinflated with BSS, you see transient blanching of the aqueous veins, indicating a patent post-trabecular outflow system.3 In comparison, our method using trypan blue mixed into viscoelastic during canaloplasty makes it much easier to visualize the pattern, distribution and caliber of the aqueous veins. Furthermore, some surgeons have used fluorescein to observe outflow fluid movement,4 but using trypan blue eliminates the need for special equipment to observe it. We refer to our pro-cess as “trypan blue venography,” even though the blue staining observed in some patients falls outside of the veins—which, again, suggests the presence of a lymphatic system in the sclera.
This modification of canaloplasty provides crucial information about the condition of the outflow system. If little or no outflow is seen—especially after the therapeutic effect of a canaloplasty has been applied—the outflow system may already be damaged beyond repair, and attempting to enhance the natural outflow system may not achieve the desired surgical result. In that situation, switching to an option that creates a nonphysiologic outflow system, such as a subconjunctival gel stent, trabeculectomy or tube shunt, might make more sense.
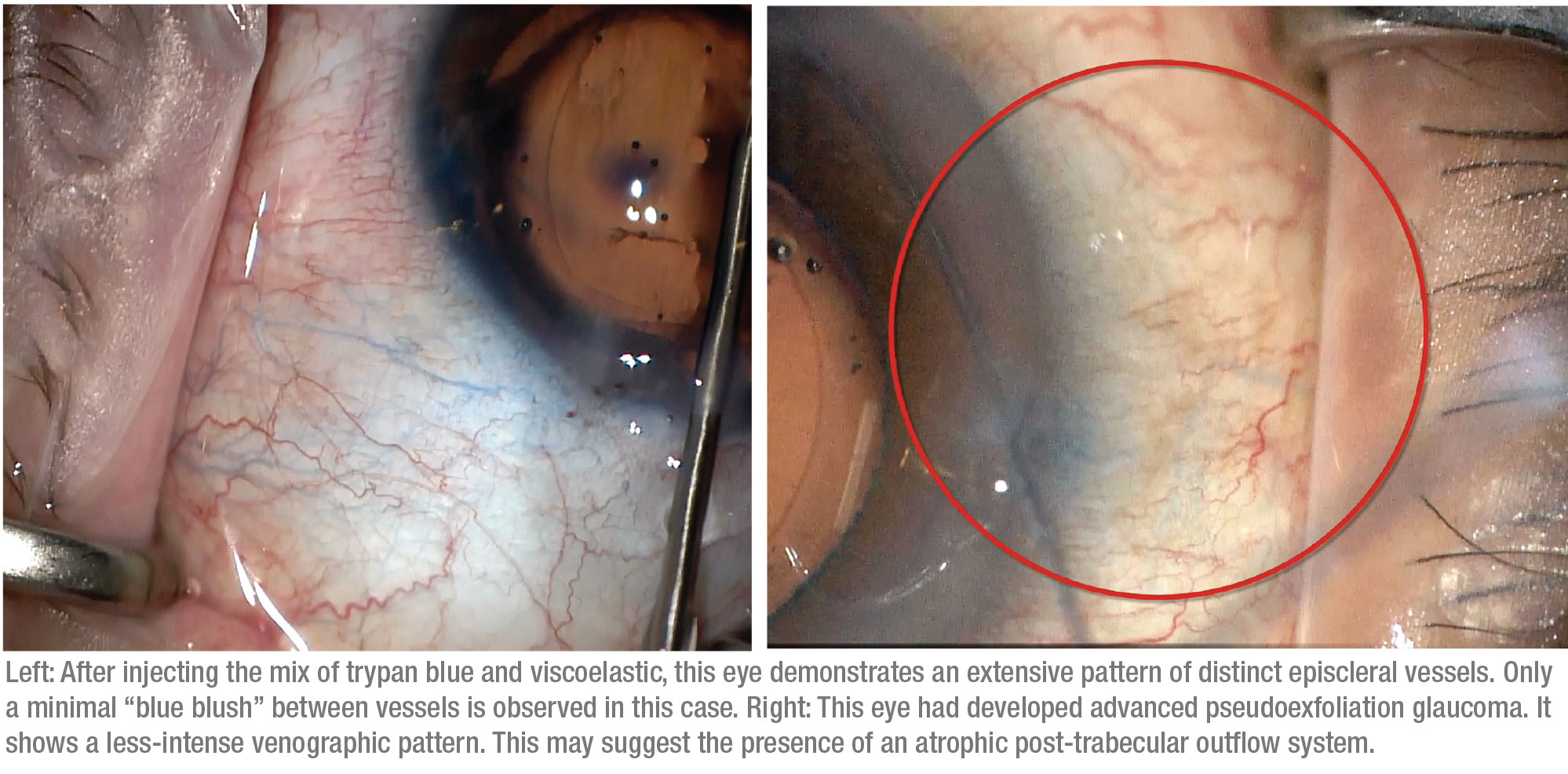
We’ve observed several different responses when trypan blue has been used in this manner. First, in some patients we see minimal-to-no trypan blue staining; these are the patients who would probably benefit more from going directly to a gel stent, tube or trabeculectomy. Second, some patients have a more sectoral or fine response, in which some areas show trypan blue staining in a limited fashion. A third group shows diffuse outflow, where we see multiple areas of vessel staining, which we feel is a favorable prognostic indicator.
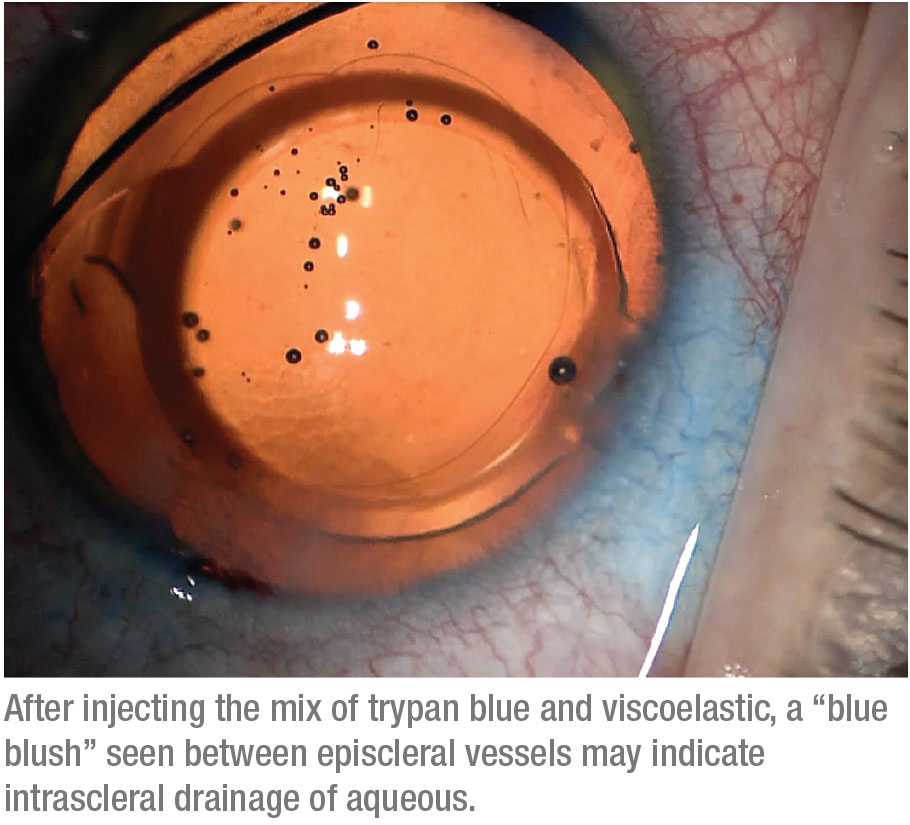
Finally, some patients show what we call a “blue blush,” where we see not just the vessels but diffuse staining within the sclera. This may indicate an intrascleral plexus that may consist of very small outflow vessels. Seeing a blue blush is probably a good sign in terms of being able to enhance the existing outflow mechanism. (Note: At this point we haven’t done enough cases to provide statistical support for an association between specific venographic patterns and different types of glaucoma.)
 |
Performing the Procedure
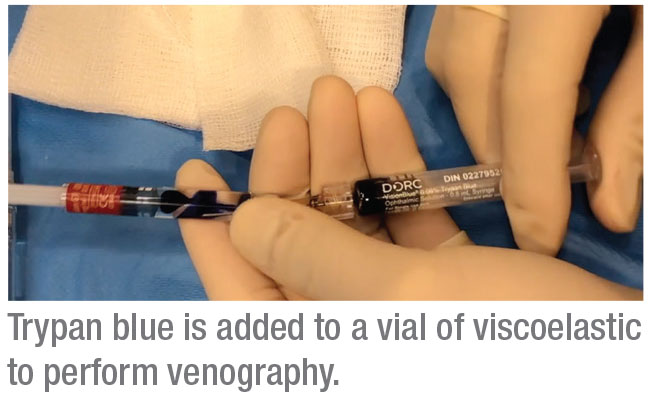
To perform this procedure, we use a cohesive OVD—Healon. These OVD cartridges are compatible with the injector mechanism for the iTrack. We inject a 0.5-mL vial of trypan blue into the container using a 1-inch, 25-gauge needle. Then we use the shaft of the needle to mix the viscoelastic and trypan blue until the mixture is as homogenous as possible. We then insert the OVD/trypan cartridge into the iTrack apparatus. The iTrack has a mechanism for injecting the viscoelastic with clockwise turns; every turn releases about 250 µm of material.
Next, we create a temporal incision, as well as a superior and/or inferior paracentesis (depending on which angle of approach we want to take), and insert the iTrack through the paracentesis. To achieve an en face view of angle structures, we tilt the patient’s head away from us and tilt the microscope about 35 degrees. We begin by filling the anterior chamber with a supercohesive OVD such as Healon 5 to create stability and prevent blood reflux from angle structures. Then, visualizing with a gonioprism, we create a small goniotomy in the trabecular meshwork using a 25-gauge needle; this acts as an entry site for the iTrack catheter. As we visualize the small goniotomy incision we’ve made in the trabecular meshwork, we insert the iTrack through it using micrograspers.
Once we’re confident that we’ve inserted the iTrack into Schlemm’s canal for two to three clock hours, we rotate the patient’s head as well as the microscope back into the primary position; this facilitates visualization of the iTrack’s fiber optic illumination and advancement through the canal. This position also enables simultaneous viewing of the post-trabecular outflow system. We proceed to inject the viscoelastic and trypan blue mixture as we carefully advance the catheter and observe the subsequent venographic pattern around the illuminated tip.
Currently, we’re cannulating and viscodilating the complete 360 degrees to assess the post-trabecular outflow system. Traditionally, most patients have a higher density of col-lector channels in the inferonasal quadrant. Interestingly, we’re find-ing a number of patients that have a higher density of collector channels in the superior quadrants. We still have much to learn about how the various venography patterns affect surgical outcomes.
Why Add GATT?
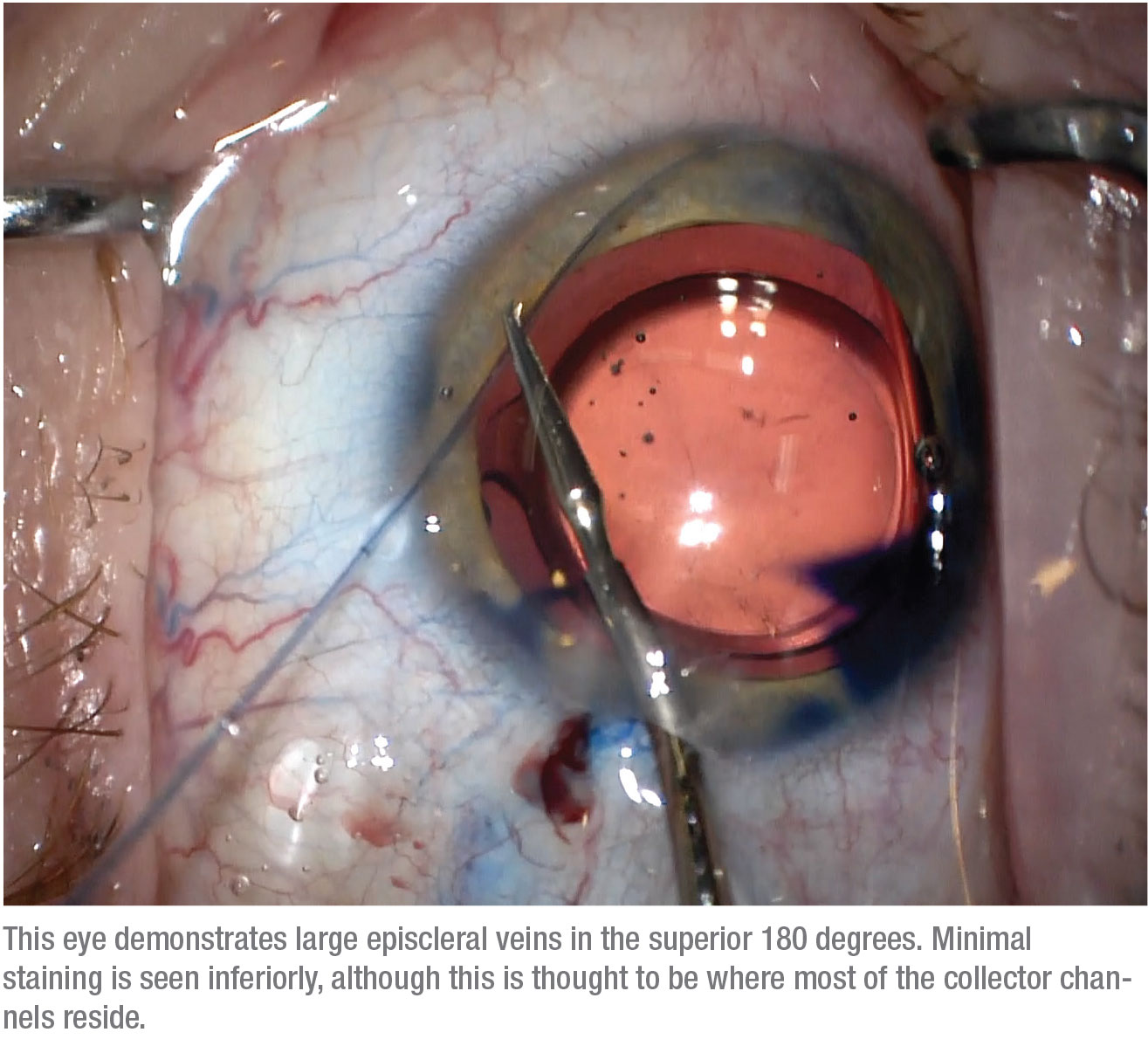 |
About 90 percent of the time we follow our modified version of canaloplasty with a GATT procedure. In the GATT procedure, Schlemm’s canal is unroofed by pulling on the iTrack microcatheter used to perform the canaloplasty while it’s still inside Schlemm’s canal. (There are some contraindications to performing the GATT procedure, such as if the patient is taking anticoagulants or has a high risk of bleeding.)
The GATT procedure was initially introduced by Davinder Grover, MD, in 2014, using a full 360-degree unroofing of Schlemm’s canal.5 In successful cases, that approach can result in a postop IOP very close to episcleral venous pressure, as low as 10 mmHg, although most of the time patients end up on fewer glaucoma medications with a postop IOP in the teens. We usually perform a hemi-GATT, meaning 180 degrees of treatment; our data suggests that this may be just as good (or almost as good) as doing a full 360 degrees, and it may lower the risk of hyphema.
We believe that adding the GATT procedure reduces resistance at the trabecular meshwork more than canaloplasty alone, especially for cases of secondary open angle glaucoma. Furthermore, performing a targeted, segmental GATT based on the patient’s personalized aqueous venographic pattern may increase our chances of success while reducing the risk of complications, since we’re not unroofing all of Schlemm’s canal. So far, we’ve been performing a full 360-degree venography, then following with a targeted hemi-GATT in the quadrants with a better aqueous venographic pattern (i.e., having more numerous vessels of larger caliber). Even if the trypan blue indicates limited functionality of the collector channels, we still tend to perform an inferior hemi-GATT, traditionally where there’s a higher density of aqueous veins. We’ve found that even in these cases it may provide some benefit.
In terms of supporting data, we have a case series that has been accepted to the Journal of Glaucoma for publication. The data so far suggests that the double procedure is effective; it produces a statistically significant amount of pressure reduction, and a significant reduction in the number of medications patients need, including a decreased need for acetazolamide, an oral carbonic anhydrase inhibitor that can be used as a last resort for lowering pressure in severe cases.
What’s Next?
In addition to accumulating a larger database from which to draw conclusions about the dual procedure’s effectiveness, we’re hoping that the patterns of staining that appear may turn out to be connected to specific types of glaucoma; this may allow us to refine treatment based on the patterns we find.
It would also be helpful to develop a way to visualize the patency of the outflow system before performing the canaloplasty. That would allow us to show how much performing a canaloplasty affects the aqueous outflow patterns, and also guide us in terms of knowing which patients would benefit from this type of procedure, and which patients have a totally sclerosed or scarred physiologic outflow system and would be best treated with a gel stent, trabeculectomy or tube shunt. REVIEW
Dr. Gooi is an associate professor in the department of surgery at the University of Calgary in Alberta, Canada, where Dr. Docherty’s work on this project was also conducted. Dr. Gooi has consulted for Aerie, Allergan, Bausch + Lomb, Glaukos, Labtician, Salient and Santen. Dr. Docherty reports no financial conflicts.
1. Xin C, Wang RK, Song S, et al. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp Eye Res 2017;158:171-186.
2. Yücel YH, Johnston MG, Ly T, et al. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp Eye Res 2009;89:5:810-9.
3. Fellman RL, Grover DS. Episcleral venous fluid wave: Intraoperative evidence for patency of the conventional outflow system. J Glaucoma 2014;23:6:347-50.
4. Akiyama G, Saraswathy S, Bogarin T, et al. Functional, structural, and molecular identification of lymphatic outflow from subconjunctival blebs. Exp Eye Res 2020;196:108049.
5. Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014;121:4:855-61.




