The number of glaucoma surgeries that could be considered MIGS expands every year. In theory, at least, that makes the choice of a MIGS procedure more challenging simply because the number of options keeps growing. Given this broadening array, two questions come to mind: First, which MIGS procedures—and how many—should you learn? And second, if you have more than one MIGS option to offer your patients, how do you choose the best one for a given patient?
To answer either of these questions, we need to clarify two things: First, what qualifies a procedure to be considered a MIGS procedure? And second, how can we subdivide MIGS into more manageable categories to simplify the choice?
MIGS: An Overview
“One of the defining characteristics of MIGS procedures is that they use an ab interno approach,” says Ronald L. Fellman, MD, attending surgeon and clinician at Glaucoma Associates of Texas, adjunct clinical professor of ophthalmology at North Texas Eye Research Institute and clinical associate professor emeritus at the University of Texas Southwestern Medical Center in Dallas. “This is obviously very different from our past glaucoma surgeries, which were all ab externo. Also, all MIGS must have a very high safety profile. In days past we only had one or two glaucoma procedures, and we were more likely to talk about the risk of surgery, which was considerable. That’s why we didn’t resort to surgery until we were convinced the patient would go blind without it. It’s a very different mindset today.”
Like many glaucoma surgeons, Dr. Fellman subdivides the MIGS procedures into categories based on the spaces that they target—although surgeons may disagree about whether every category qualifies as MIGS. “First is the trabecular space, including Schlemm’s canal, which is the conventional outflow path,” Dr. Fellman says. “Today, the device most commonly used in the trabecular meshwork is the iStent, but others in that space include the Trabectome, the Kahook Dual Blade and circumferential trabeculotomy, with or without viscodilation. The Hydrus should also be available pretty soon, as well as new iStent models. Second is the supraciliary space, for which only the CyPass is currently approved. Third is the subconjunctival space. The only device currently approved for that is the XEN, with the InnFocus on the horizon.”
Dr. Fellman notes that there’s some disagreement among surgeons regarding whether it’s legitimate to categorize subconjunctival procedures such as the XEN as MIGS surgeries. “Some people call these procedures ‘MIGS-plus,’ but I think it’s reasonable to consider the XEN a MIGS procedure because it’s very different from a standard trabeculectomy,” he says. “We’re hoping that the XEN, which has a set internal lumen size of 45 µm, will produce a more manageable, standardized bleb. However, it’s not that simple. A third of the people implanted with a XEN have to be needled, usually in the office. The reality is, most general ophthalmologists don’t want to mess with a bleb, so they tend to stay away from the
 |
subconjunctival space, and if you’re not comfortable dealing with a bleb you shouldn’t be doing the XEN. Furthermore, in the MIGS arena, any subconjunctival procedure is going to be less predictable than some of the other MIGS procedures. Nevertheless, I believe it is legitimate to consider the XEN a MIGS procedure.
“Finally, I believe endoscopic cyclophotocoagulation should be included in the MIGS group in a fourth category, as a means to decrease aqueous humor production,” he concludes. “So there are four broad spaces that you can approach ab interno: three outflow, one inflow.” (Some surgeons might include micropulse transscleral cyclophotocoagulation in the fourth category, although it has been associated with some complications that lead many to view it as not belonging under the MIGS mild, “less-invasive” umbrella.1)
Which MIGS Should You Learn?
Because so many MIGS procedures are available—with more waiting in the wings—should you learn to do more than one? And if so, how many?
Most experts agree that every ophthalmologist should be able to perform at least two—preferably three—MIGS procedures. They offer the following advice:
• Learn MIGS procedures that reduce pressure in different ways. This makes sense for at least two reasons: One, a given approach may not work with a particular patient. Two, if your first choice works but doesn’t produce sufficient pressure reduction, you’ll have a second option to add.
“Even if you’re a general ophthalmologist, if you have a favorite MIGS procedure that you’re comfortable with, you should be able to do something different in case that procedure doesn’t work or the anatomy doesn’t favor it,” says Brian Francis, MD, MS, a professor of ophthalmology in the glaucoma service and the Rupert and Gertrude Stieger Endowed Chair at the Doheny Eye Institute, part of the David Geffen School of Medicine at the University of California, Los Angeles. “If you’re a glaucoma specialist, you should be able to choose an option from three, or even all four MIGS categories. The point is that you don’t want to use one procedure for every situation. You want to have some ability to tailor the procedure to your patient.”
Dr. Fellman notes the benefits of being able to add together the efficacy of multiple MIGS. “Let’s say you do a phaco-CyPass, tapping into the uveoscleral outflow system, and it doesn’t lower the pressure enough,” says Dr. Fellman. “You could go back later and perform various trabecular outflow procedures, because many are approved as standalone procedures. Now you’ve tapped into both natural outflow systems. This approach might actually be the most effective strategy, but no one has done a study to prove that—so far. The XEN is another follow-up option in this situation, because it’s also a standalone that doesn’t have to be paired with phaco. But don’t get into the XEN unless you’re willing to be a blebologist and needle blebs.”
• Learn more than one trabecular meshwork procedure. Dr. Fellman believes it’s worthwhile to be able to perform more than one canal-based procedure. “It’s good to know not only how to bypass the meshwork with an iStent, but how to either remove it with something like the Dual Blade, or cleave it open with the GATT procedure,” he says. “They work differently, and one might be a little more aggressive than the other.”
Brian E. Flowers, MD, managing partner/glaucoma specialist at Ophthalmology
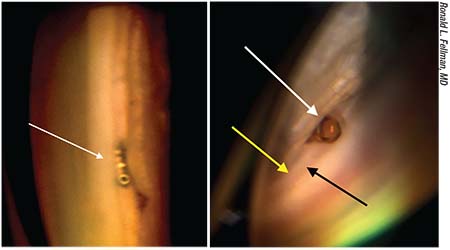 |
| Left: An iStent is well positioned in Schlemm’s canal, bypassing the diseased trabecular meshwork. Right: A CyPass, well positioned in the nasal angle (white arrow). The collar is flush with the anterior edge of the trabecular meshwork (black arrow) but inserted below the scleral spur (yellow arrow). |
Associates of Fort Worth in Texas, agrees that ophthalmologists could benefit from being able to perform two Schlemm’s canal-based procedures such as the iStent and Kahook Dual Blade—as well as one supraciliary procedure. “The iStent and Kahook Dual Blade are fairly straightforward,” he notes. “A glaucoma specialist will probably be more comfortable working in the angle, so he or she might want to master a few of the more challenging MIGS procedures as well.”
• Be able to perform a MIGS procedure that you can do without cataract surgery. “Most MIGS procedures—though not all—are approved only in conjunction with cataract surgery,” Dr. Francis notes. “You should be able to perform at least one that’s approved for use when the patient doesn’t need cataract surgery.”
• If you’re willing to work with a bleb, learn one of the subconjunctival MIGS procedures. “These appear to have a reduced risk profile compared to a tube shunt or trabeculectomy,” Dr. Francis points out. “I think it’s worthwhile to master at least one of those.”
In the final analysis, of course, each surgeon will probably find that certain MIGS procedures are more comfortable and produce better results in his or her hands. “The most important thing,” says Dr. Fellman, “is to use the MIGS procedures that you’re most comfortable with, the ones that work the best for you.”
Mastering MIGS
Once you’ve decided which MIGS you’d like to add to your armamentarium, these strategies can help to smooth your path:
• At the outset, stick with one procedure that you want to learn. “If you’re not just concentrating on one procedure, you’re not giving yourself the best opportunity to learn it well,” says Dr. Fellman. “It’s a bad idea to say, ‘Let me try this, let me try that.’ Commit to learning one procedure, get it down, and then move on.”
• Practice the positioning and maneuvers before attempting the surgery. “Performing MIGS requires a very different proprioceptive feedback loop on the part of the surgeon,” notes Dr. Fellman. “When performing something like phaco, you’re looking through the microscope at the tips of the two instruments in your hands. Your brain becomes accustomed to knowing where your hands are, based on that view. But that proprioceptive loop is gone when you work in the angle. You have to establish a new mental feedback loop, and that takes a while.
“To work in the angle, you have to turn the patient’s head, and you have to turn the microscope,” he continues. “Nobody’s initially used to working in that position. Then, you have to learn how to put the gonio lens on the eye so that you balance it without excessive pressure. Over the years I’ve worked with many surgeons learning to do angle procedures, and the most common initial problem is a poor view because of excessive pressure on the cornea with the nondominant hand.”
Dr. Fellman says the way to iron out these issues before attempting surgery is to practice these movements on patients who don’t need a MIGS procedure. “You can practice these maneuvers with some of your glaucoma patients when you’re performing phaco,” he says. “At the end of the procedure—or at the beginning if that makes you more comfortable—you can turn the patient’s head away from you and turn the microscope towards you and use the Swann-Jacob gonio lens (which is a lens, not a prism) and see what you can see. This will give you a chance to experience what it’s like to balance a gonio lens on the eye with your nondominant hand and do it well enough to maintain a pristine, excellent view of the chamber angle.
“Then, with your dominant hand, make a few simple motions,” he continues. “You can even put an instrument in your hand just to feel what it would be like if you were working in the angle. But the important part is to practice that until you get a really good view. That will give you a leg up when you do your first MIGS case.”
• Take advantage of educational resources. “Look at lots of videos,” advises Dr. Fellman. “Most device makers have a course you can take and some lab models you might be able to use. If you’re lucky enough to be in an area where someone is already doing the procedure, you may
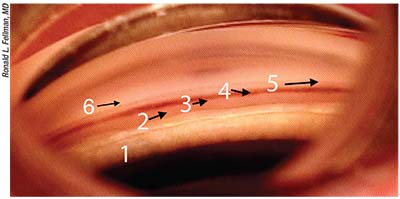 |
| A good understanding of angle anatomy is crucial to performing MIGS. 1) Area close to pupil. 2) Ciliary body band. 3) Scleral spur. 4) Trabecular meshwork. 5) Schwalbe’s line. 6) The area above Schwalbe’s line, where a XEN would typically be placed. |
want to go and watch.”
Dr. Flowers notes that the companies tied to MIGS procedures provide a lot of resources to help surgeons master them. “They offer wet labs and will even send someone into the OR with you as a mentor,” he points out. “In addition, they offer access to experienced MIGS surgeons on a consultative basis for questions and issues with individual patients. Probably the most important thing is to brush up your gonioscopy skills and make sure you’re comfortable with what you’re seeing. Practice gonioscopy on a regular basis. That can’t be overstated.”
“All those things come together in the end to make you a little more confident when you go into the OR,” Dr. Fellman says. “As you know, confidence is always important to a surgeon. You don’t want to go into the OR feeling unsure about whether you can do this. Your frame of mind is just as important as the frame of the angle! So pick out one MIGS procedure to start, practice the type of positioning and movements you’ll need in surgery, and take advantage of every educational resource you can. You’ll be off to a great start.”
Which MIGS for Which Patient?
Once you’ve learned to perform two or more MIGS, you’ll have to decide which MIGS makes the most sense for the patient you’re currently managing. The choice you make will depend on a number of factors, ranging from the patient’s angle anatomy to your comfort with the surgical requirements of a given option. When making a choice, the following 12 strategies will help:
1. Know your anatomy. “The area of concern with MIGS procedures is the iridocorneal angle, which goes from the iris to the cornea,” Dr. Fellman points out. “The picture above shows the different parts of the angle anatomy clearly. The area marked 1 is close to the pupil. To do an ECP, you go in behind the iris with your ECP probe and locate the ciliary body processes. Number 2 marks the ciliary body band, which attaches to the scleral spur. Number 3 is the scleral spur, a vital landmark that divides up the iridocorneal angle and determines where you’re going to place your MIGS. Uveoscleral outflow occurs below, or posterior to, the scleral spur, going out through the ciliary body, while trabecular outflow occurs above the spur (the trabecular meshwork, labeled 4). So the scleral spur differentiates where the iStent and CyPass go.
“In this picture the meshwork has pigment, about 3+,” he continues. “This is where an iStent would be placed, and the location for the Dual Blade, Trabectome, trabeculectomy-viscodilation and GATT. Number 5 is Schwalbe’s line; that’s where the cornea starts. Right above Schwalbe’s line—the area labeled 6—is where you typically want to place your XEN.”
2. Keep your gonioscopy skills in top shape. “To perform MIGS, you have to be comfortable working in the angle,” notes Dr. Francis. “You have to be comfortable with operative and preoperative gonioscopy. Knowing the anatomy in that space is key to identifying which procedure to do, and to targeting the correct tissue during the procedure.”
Dr. Fellman agrees. “Being able to identify and visualize the structure that your procedure targets is obviously crucial to a successful surgery,” he says. “Because the angle anatomy is so important, in order to successfully perform MIGS a surgeon has to be more than just good at gonioscopy; you have to be a superb gonioscopist.
“I find that many comprehensive ophthalmologists—and even glaucoma specialists—who are really interested in MIGS haven’t done enough gonioscopy,” he adds. “That’s not entirely surprising, because when you do a trabeculectomy or a tube shunt, gonioscopy is not as important as it is with MIGS. As a result, gonioscopy is underutilized. In contrast, gonioscopy is critical to the success of all of the MIGS outflow procedures.”
3. Focus on the most important factors. If you’re not yet certain about which things you should pay attention to when choosing a MIGS procedure for your patient, Dr. Fellman suggests using the acronym “ABCDE” to remember five key factors.
“You can say that ‘A’ stands for the angle,” he explains. “The key landmark in the angle is the scleral spur, which separates the trabecular outflow area from the uveoscleral outflow area. That’s important to be able to see and identify, because different MIGS procedures require access to one space or the other. Identifying these locations and determining how well you can see them will help you decide what you can do safely in a given eye.
 |
“ ‘B’ stands for the blood-aqueous barrier,” he continues. “The condition of the blood-aqueous barrier helps reveal the integrity of the environment inside the anterior chamber. For example, if you have a lot of flare and cell, that’s not a good sign for wound healing. You wouldn’t want to do any kind of filtering surgery—including the XEN—in an eye like that. You need a pristine blood-aqueous barrier to have a good shot at successfully implanting a XEN.
“ ‘C’ stands for the conjunctiva,” he says. “If the conjunctiva is damaged or scarred, that also should lead you away from a filtering procedure and towards a trabecular or uveoscleral approach.
“ ‘D’ represents the disc,” he continues. “Many of the MIGS procedures are only approved for mild to moderate glaucoma damage, so if the patient’s nerve is badly damaged you’ll want to use a procedure that will give you a lower pressure in a more predictable fashion. This is especially important if you’re just learning one of the MIGS procedures; that surgery is not going to be as predictable as a procedure you’ve been doing for years, such as a standard trabeculectomy or tube shunt.
“ ‘E’ stands for your level of expertise,” he concludes. “Of course, there’s a learning curve to everything, but you can reduce the learning curve by getting certified, by watching other surgeons in the OR who’ve done the procedure a lot, and by watching online videos.”
4. Be alert for scarring. “If you have conjunctival scarring from a scleral buckle or other cause, then you’re going to want to steer away from the subconjunctival filtration procedures, such as XEN or InnFocus,” notes Dr. Francis. “If you have scarring in the angle from, say, chronic angle closure, then you may want to stay away from the trabecular outflow procedures. Instead, you might go with something that decreases aqueous production, or go with subconjunctival filtration. You have to take into consideration what the anatomy is giving you.
“In most patients, knowing the condition of the collector channels before surgery is difficult or impossible, at least with today’s technology,” he continues. “However, you might encounter a patient with severe scarring that you know will affect the collector channels, whether as a result of chemical trauma or a disease such as ocular cicatricial pemphigoid. In that situation, you’d want to steer away from trabecular-outflow-enhancing procedures.
“This gets to the concept of targeting,” he says. “Let’s say you have a device that only accesses a certain portion of the angle, such as an iStent or Hydrus. In that situation you’d want to try to identify target collector channels by determining where the outflow already exists and can be recruited. On the other hand, with a supraciliary stent like the CyPass, you’re not as worried about collector channels because you’re not using them. The same should be true for decreasing aqueous humor production and subconjunctival filtration.
“It’s worth noting that the MIGS procedures that unroof Schlemm’s canal are a little different from the stents, because you’re accessing more of the angle,” he adds. “For instance, the Trabectome, the Goniotome and the Kahook Dual Blade all treat about 180 degrees of the angle, and the Trab 360 and GATT procedures both treat 360 degrees of the angle. With those procedures, there’s less need to worry about targeting specific collector channels and outflow pathways.”
5. For an eye with a narrow angle or plateau iris, consider using ECP. “Narrow angles are not a big deal when performing most MIGS procedures that are approved with cataract surgery,” notes Dr. Flowers. “Once you take the cataract out, the angle is no longer narrow, allowing you to perform any of the MIGS procedures. Of course, doing so would be off-label, because these procedures are not specifically approved for narrow-angle cases, but they would certainly work in that scenario.”
Dr. Fellman notes that many surgeons do phaco-ECP in a narrow-angle eye. “Narrow-angle eyes are more prone to postop complications such as aqueous misdirection that can occur when the pressure gets too low,” he points out. “Phaco-ECP is a very safe way to handle a narrow-angle eye because by deepening the chamber with phaco you’re going to increase the outflow and lower the pressure, while the ECP will decrease the inflow. This is also very effective in eyes with plateau iris, because you can do the ECP a little bit more anteriorly to shrink the peripheral iris and open up the angle a bit more.
“You can do even more for a narrow-angle patient by performing goniosynechialysis,” he continues. “If you encounter four or five clock hours of peripheral anterior synechiae, which is not uncommon in these eyes, you can push the iris posteriorly and break those adhesions during the surgery. That’s going to open up the angle as well. Now you have three mechanisms that are lowering the pressure: the phaco, by deepening the chamber; the goniosynechialysis, by opening up the meshwork to the flow of aqueous; and finally the ECP, which is reducing aqueous inflow.”
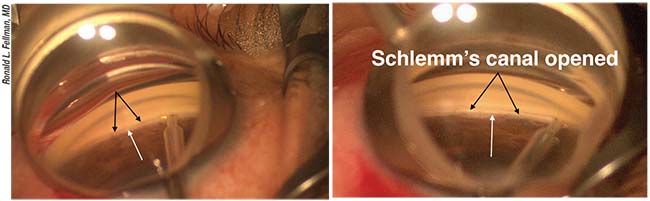 |
| Left: A Trabectome positioned adjacent to the trabecular meshwork (black arrows). The white arrow points to the scleral spur. Right: The Trabectome has removed part of the trabecular meshwork and inner wall of Schlemm’s canal. The white stripe (black arrows) is the back wall of the canal; the white arrow denotes the scleral spur. The Trabectome is then aimed in the other direction to ablate the remaining TM. |
However, Dr. Flowers notes that PAS are not always breakable. “PAS are only easy to break if they’ve been in the angle for six months or less,” he says. “After that, they can be difficult or impossible to break, and that will definitely make most MIGS procedures impossible to perform. Certainly a XEN procedure would be impossible under these conditions.”
6. Consider how much pressure reduction the patient needs. “In most cases, the more severe the disease, the lower your target pressure will be,” says Dr. Francis. “If you’re trying to reach a target pressure in the low teens, for example, you don’t want to be limited by episcleral venous pressure or other physiologic constraints. You’re probably going to want to consider a subconjunctival filtration procedure. If you’re not going to do one of those, then you might want to combine multiple other MIGS procedures. You could combine a suprachoroidal shunt and a procedure that reduces aqueous humor production; or combine a trabecular meshwork procedure with aqueous humor reduction.”
“In glaucoma,” notes Dr. Flowers, “one of the axioms is, ‘The more you do, the more you get.’ For example, if you’re working in the trabecular meshwork, you might be debating whether to implant an iStent or a Hydrus. [The Hydrus has completed its clinical trials, but it hasn’t been approved as of this writing.] The iStent is a small device that opens up a very localized segment of the trabecular meshwork, while the Hydrus opens up an entire quadrant of Schlemm’s canal. Our group participated in the Hydrus clinical trial, and the data was just published; its IOP-lowering effect appears to be greater than that achieved with one iStent. Of course, the caveat is that the trial designs weren’t exactly the same. Nevertheless, based on our best interpretation of the data, the Hydrus has a better long-term IOP effect that a single iStent does. That goes along with the idea that the more you do, the more pressure reduction you get.
“Generally, the same could be said for the Kahook Dual Blade vs. the GATT procedure,” he continues. “The Dual Blade opens up a limited portion of Schlemm’s canal, whereas a GATT-type procedure opens up the entire canal and can potentially give you a better pressure effect. Of course, that’s a supposition, because there’s no head-to-head study comparing the two. However, I think most surgeons would expect to achieve a greater effect by opening up the entire trabecular meshwork than just part of it.”
In terms of comparing options that affect different outflow pathways, Dr. Flowers notes that the clinical studies done to test the CyPass and Hydrus implants had almost identical designs. “Because the designs were so close, I think you can draw some comparisons there,” he says. “They showed pretty similar IOP-lowering effects. Of course, it’s not a perfect comparison, because they weren’t in the same trial together.”
Regarding balancing safety and efficacy, Dr. Flowers says that each comparison of MIGS procedures is different. “It might be true that the GATT procedure carries a little more risk than the Kahook Dual Blade,” he says. “The more angle you disrupt, the greater your risk of bleeding in the short and long term. On the other hand, I’d say that’s probably not true for the Hydrus vs. the iStent, because the risk of those two procedures appears to be about the same.”
Dr. Flowers points out a seldom-discussed consideration relating to your choice of MIGS when a more significant drop in pressure would be ideal. “One important distinction between a supraciliary procedure and the trabecular-meshwork-based MIGS is that some patients will have a dramatic drop in pressure with a ciliary body procedure—the kind of drop you’ll never see with the trabecular meshwork options. This is easy to miss if you only look at the mean IOP-lowering data. In the CyPass IDE trial, for example, the average pressure lowering was 2 mmHg greater than was achieved with cataract surgery alone—but some patients had much more significant drops in pressure.2 That means that this type of procedure at least has the potential to have that kind of impact.
“At the moment we don’t know why some patients have that more dramatic response, although one would think that it has to do with the healing response,” he continues. “When you do a supraciliary implant like a CyPass, you’re creating a dialysis—ideally a limited dialysis—and then a healing process takes place. As with many surgeries, if it heals over too aggressively, the function decreases or is eliminated. So, patients who keep their limited dialysis open for an extended period of time will have a more dramatic, sustained drop in their pressure.”
Dr. Flowers says he keeps this possibility in mind when choosing a MIGS procedure. “If I’m trying to achieve a significant drop in pressure, I know a supraciliary procedure at least has the potential to achieve that, for a minimal—possibly even theoretical—increase in risk,” he says. “That’s definitely a factor in my decision process.”
Of course, it’s also possible to do more than one MIGS procedure at the same time in order to achieve a larger pressure reduction. Dr. Flowers says that with only a few exceptions, however, he prefers not to perform multiple MIGS simultaneously. “So far, I’ve only done that in one circumstance,” he says. “I had a patient with chronic inflammation and multiple failed filtration surgeries. We went in and did a GATT plus ECP, and that controlled his pressure. So there are situations in which doing ECP plus some kind of angle procedure can be beneficial in certain populations.”
7. Aim for the safest option. “Ultimately, the reason MIGS exists is for safety,” Dr. Flowers notes. “Every glaucoma procedure has an efficacy side and a safety side, and there’s a continuum of surgeries that range from being highly effective pressure-reducers that carry many more safety risks, to those that are incredibly safe but produce nominal pressure benefit. I’d argue that the relationship is not linear, however—meaning there are surgeries that can achieve slightly better pressure benefit without changing the safety factor very much.
“For me, the deciding factor when I’m choosing a MIGS procedure is the safety side first, although both factors matter,” he concludes. “I consider how much I believe this particular patient can tolerate in terms of risk, and then balance that against the amount of pressure reduction I need to get. I pick the MIGS procedure that I believe yields the best balance—but I do lean towards safety first.”
8. Consider the patient’s age. Dr. Francis notes that the age of the patient can influence the surgeon’s choice of MIGS procedures for a couple of reasons. “If the patient is very elderly, you might choose to be less aggressive about lowering their pressure,” he says. “You may want to stay away from the transconjunctival procedures and go with a more traditional MIGS. On the other hand, age also decreases the amount of scarring you encounter. A younger patient would be more likely to produce a lot of scarring with a transconjunctival procedure such as XEN, so that might not be as appropriate in a young patient.”
Dr. Flowers says the age of the patient doesn’t influence his choice of MIGS procedure, except in one respect. “In glaucoma it’s always important to be planning for the next procedure, because every procedure has a lifespan that follows the Kaplan-Meier curve,” he says. “If you do 100 procedures, 90 percent of them will be working a year later. At two years, 80 percent of them will be working, and so on. So, you always want to be thinking about not foreclosing future opportunities by creating conditions that will make it impossible to do the next procedure the patient may need. That’s one of the main reasons I’m not a big primary tube shunt guy. Once you stick a tube in the eye, you’re starting to foreclose future opportunities for other surgeries.”
Although MIGS seem relatively harmless in terms of reducing future options, Dr. Flowers points out that the GATT procedure, which disrupts the entire trabecular meshwork, would eliminate any future possibility of putting a device into Schlemm’s canal. “For example, if the Hydrus or future-generation iStents become available and you’ve already done a GATT, you won’t be able to use them,” he says. “That definitely makes me hesitate to choose a MIGS procedure that’s going to destroy the trabecular meshwork.”
Dr. Fellman notes that the age of the patient is a double-edged sword. “The longer you’ve had glaucoma—especially being on drops for decades—the less likely it is that your collector channels will be salvageable,” he says. “You may think you’re not going to get much flow through the patient’s natural drainage system because they’ve had the disease for so long. On the other hand, it’s a pretty simple thing to add on a trabecular MIGS procedure, and there’s very little downside to it; the safety issues are minimal, and every point of IOP reduction you can obtain may be meaningful. Also, if you’re thinking about a XEN, older patients may actually do better because they’re less likely to scar. It’s still unpredictable, but young patients definitely scar more.” (Dr. Fellman adds that the episcleral venous fluid wave, seen in the operating room as a clue to outflow capacity, is a very helpful hint regarding the postoperative IOP outcome.)
9. Don’t abandon MIGS because a previous one didn’t work. “If one MIGS procedure has failed to produce the desired pressure reduction, I’d try a MIGS in a different category,” says Dr. Francis. “For example, if you’ve done an iStent and it didn’t work, I wouldn’t abandon MIGS. You could take out the iStent and do a Trabectome or KDB, or use ECP to decrease aqueous production. Or, if you’ve done a KDB or Trabectome or Goniotome, you could add a CyPass or perform ECP. You just want to try something that works in a different way from what you’ve already tried.”
10. Don’t worry about which medications the patient has been on. “I don’t think it makes a difference which medications the patient has been on,” says Dr. Francis. “The efficacy of drops and surgery can be very different. For example, surgery to reduce aqueous humor production might be far more effective than drops that have that effect.”
Dr. Flowers agrees that the impact of a drop on a given outflow pathway is going to be tiny compared to the impact of a MIGS procedure. “It’s not even in the same ballpark,” he says. “A drop might give you a tiny bit of enhanced flow, but even a tiny opening in the ciliary space, for example, will allow a deluge of fluid to pass through compared to what the drop accomplishes. The ultimate issue is how long the outflow pathway stays open.”
11. Avoid using procedures off-label. “There’s more and more data coming out that these procedures are very efficacious as standalone procedures, but from an insurance and surgery-center-logistics perspective, going off-label is a nightmare,” says Dr. Fellman. “Most patients can’t afford to pay for these procedures. You’re talking about a surgery center bill, an anesthesiologist bill, a surgeon bill and a device bill. That’s going to add up to thousands of dollars.”
Dr. Francis agrees. “Whether or not the patient has visually significant cataracts is very important from a logistics standpoint,” he says. “Some devices, such as the iStent, Hydrus and CyPass, are only FDA-approved for implantation at the time of cataract surgery. There’s no reason these devices wouldn’t work in a pseudophakic patient, but it wouldn’t be on-label because those are only FDA-approved for use with cataract surgery. It’s more of a logistical issue; if you do a procedure off-label, you won’t get paid. That’s not a small consideration for most surgeons. Your insurance claim will be denied and you’ll have to charge the patient cash.”
“Fortunately, a few MIGS procedures are approved for use apart from cataract surgery,” Dr. Fellman adds. “The Dual Blade and Trabectome, for example, are approved for use as standalone procedures, so they can handle those cases that don’t have to be done in combination with phaco.”
12. Remember that a MIGS procedure can be effective even after a failed trabeculectomy or tube. “Following a trabeculectomy, you’ve abandoned the patient’s natural drainage system,” Dr. Fellman points out. “Let’s say you did a trabeculectomy or tube on a phakic patient. Then the patient eventually develops a cataract and the trabeculectomy slowly fails. You could easily do a phaco-iStent, or phaco-Trabectome, or Dual Blade or CyPass. There’s a decent chance that this eye will then end up with a pressure low enough that the patient does OK. In fact, our group has shown that the GATT procedure, which opens up 360 degrees of the trabecular meshwork and the inner wall of Schlemm’s canal, is very effective in eyes that already had a trabeculectomy or tube.3 In other words, you don’t have to abandon the patient’s natural drainage system because of a prior filtering procedure. You might be able to increase the flow capacity of the patient’s natural drain with MIGS surgery even after a failed tube or trabeculectomy.” (Dr. Francis notes that previous laser trabeculoplasty doesn’t seem to impact the efficacy of MIGS, either.)
Dr. Fellman adds that even a XEN, which shares some characteristics with a trabeculectomy or tube, can work when a trabeculectomy or tube has failed. “We’ve had many patients in that category who now have a successful XEN,” he says. “In this situation you have to make sure the conjunctiva is in adequate shape, meaning that it’s mobile over the area where you’re going to put the XEN. But there’s less trauma to the tissues associated with a XEN than with a trabeculectomy—even using mitomycin-C—and the flow is more standardized through the XEN. Of course,” he adds, “you still have to be a blebologist, and you still have to be able and willing to needle the eye if scarring is more excessive than you anticipated.” REVIEW
Dr. Francis is a surgical trainer for NeoMedix and consultant for Glaukos and BVI Endo Optiks; he has received research support from Allergan, InnFocus, Iridex and Ivantis. Dr. Flowers has participated in most of the MIGS clinical trials; he has consulting relationships with Alcon, Glaukos, Ivantis, Sight Sciences and InnFocus. Dr. Fellman is a consultant for Endo Optiks.
1. Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: Initial results in refractory glaucoma. J Glaucoma 2017;26:8:726–729.
2. Vold S, Iqbal IK, Craven ER, et al. Two-year COMPASS trial results: Supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmol 2016;123:10:2103–2112.
3. Grover DS, Godfrey DG, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy in eyes with prior incisional glaucoma surgery. Journal of Glaucoma 2017;26:41-45.



