Conjunctival dehiscence or retraction usually heals on its own with time; however, when the dehiscence exposes an underlying glaucoma drainage device, immediate repair is needed to reduce the chances of further complications such as hypotony and endophthalmitis.1,2
Wound dehiscence may occur in the early postoperative period, and erosion may occur in the late postoperative period.2 Repairing the conjunctiva after a tube exposure is challenging, especially if the patient has undergone previous conjunctival surgeries. Here, I’ll share a case example and describe my approach to conjunctival repair after tube exposure.
Risk Factors
Patient risk factors for conjunctival dehiscence include older age, high doses of topical and oral steroids, tobacco use and prior conjunctival surgeries. Immune mechanisms may also play a role.3
Dehiscence risk after GDD implantation can be decreased by placing the tube superiorly. In a small study of patients who received Ahmed glaucoma valves (n=158),4 the inferonasal quadrant was associated with the highest dehiscence rate (4/7, 57.1 percent), followed by the inferotemporal quadrant (30/65, 46.2 percent), the superotemporal quadrant (15/61, 24.6 percent) and the superonasal quadrant (4/25, 16 percent) (p<0.0073).
This same study also reported that using a greater number of preoperative hypotensive medications affected dehiscence rates. The 91 (57.6 percent) subjects who didn’t experience complications used an average of 3.3 hypotensive medications before surgery, compared with 3.8 and 3.9 medications used by the dehiscence and exposure groups, respectively (p=0.01).
In two cases reported in the literature, wound dehiscence and Ahmed valve exposure occurred with the use of adjunctive subconjunctival bevacizumab injection.5
 |
Case Example: Tube Exposure
An 82-year-old female patient was referred to my colleague with a shallow anterior chamber and high pressure. Two months prior, she’d undergone pars plana vitrectomy for vitreous hemorrhage. Her visual acuity in the right eye was counting fingers and her pressure was 25 mmHg. Medications included timolol, dorzolamide, brimonidine and latanoprost as well as acetazolamide pills and atropine.
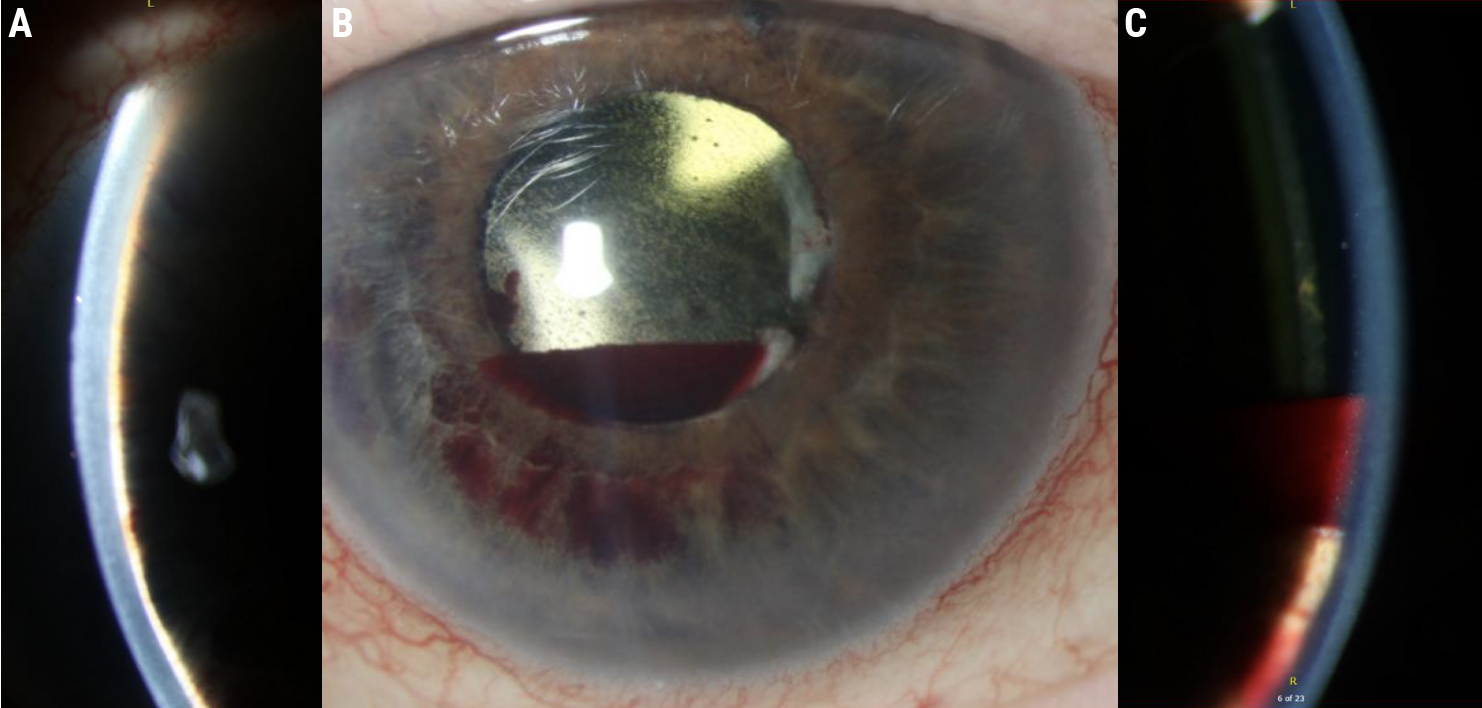
Slit lamp exam showed an extremely shallow anterior chamber with extensive iris-cornea touch (Figure 1A), and a small hyphema settling between the cornea and lens implant (Figure 1B, C). Fundus exam showed a normal disc (cup-to-disc ratio: 0.4), drusen and normal periphery.
She was diagnosed with aqueous misdirection due to retained vitreous skirt and subsequently underwent pars plana vitrectomy with an Ahmed tube placed through the pars plana. On postoperative day one, her anterior chamber had reformed and IOP was 14 mmHg. At postoperative week one, her IOP was 13 mmHg and she developed large serosanguinous choroidals. It’s common after a sudden pressure drop for fluid to collect in the suprachoroidal space. In case these effusions had an inflammatory component the patient was treated with high-dose steroids, both oral (prednisone) and topical (prednisolone acetate).
Normally, the conjunctival surgical incisions would begin to scar and heal over time, but a high steroid dose can inhibit healing. This likely contributed to the split incision seen in Figure 2. If you look carefully, you can see the purple 8-0 Vicryl suture is still intact in the conjunctiva after cheese-wiring through the anterior edge of the conjunctiva. Surgical repair was performed. Intraoperatively, an additional patch graft was placed but there wasn’t enough conjunctival mobility to achieve closure (Figure 3).
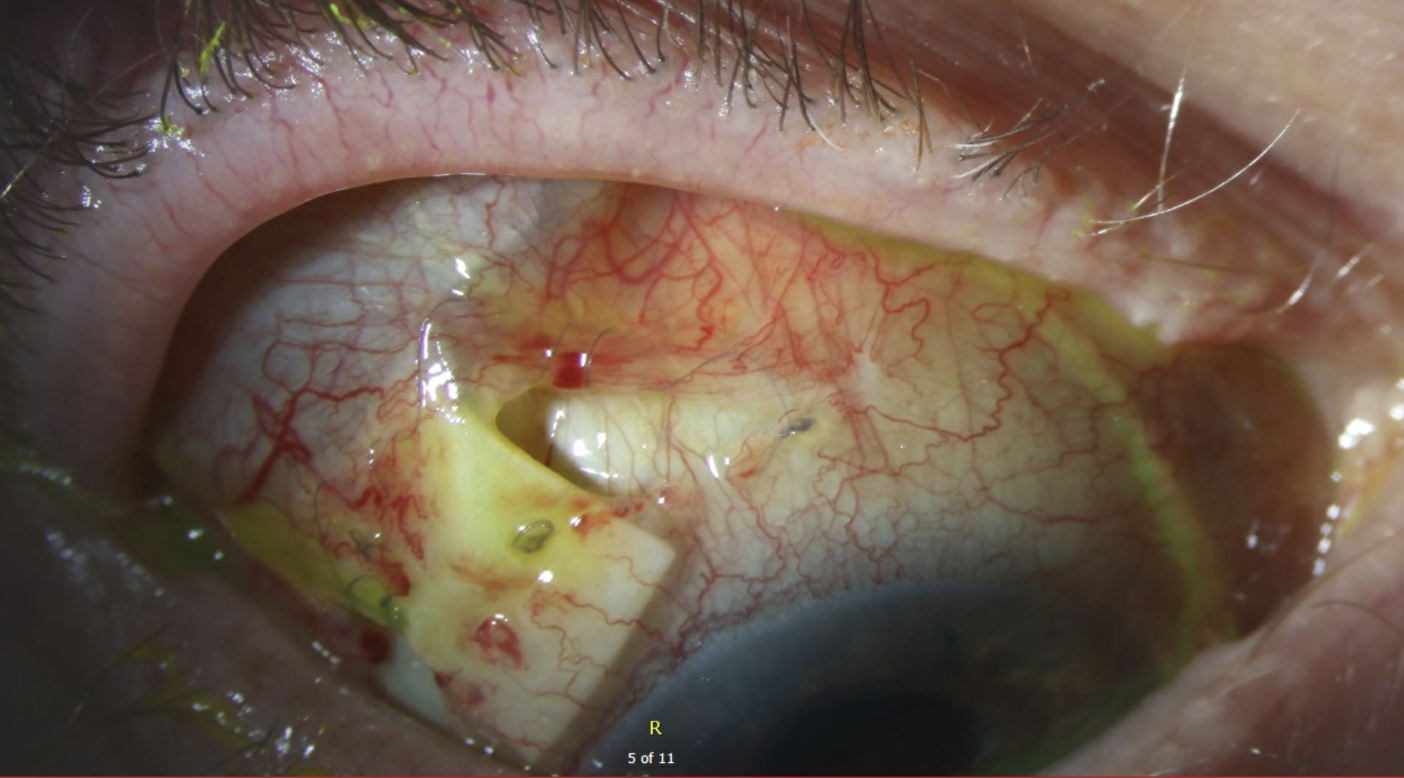 |
| Figure 2. The patient’s eye on postoperative week three, following a pars plana vitrectomy to remove retained vitreous skirt and Ahmed tube placed through the pars plana. High-dose prednisolone and prednisone were initiated for serosanguinous choroidals. The steroids likely resulted in this dehiscence. Note the 8-0 Vicryl braided sutures (purple) have cheese-wired through the anterior edge of the conjunctiva. |
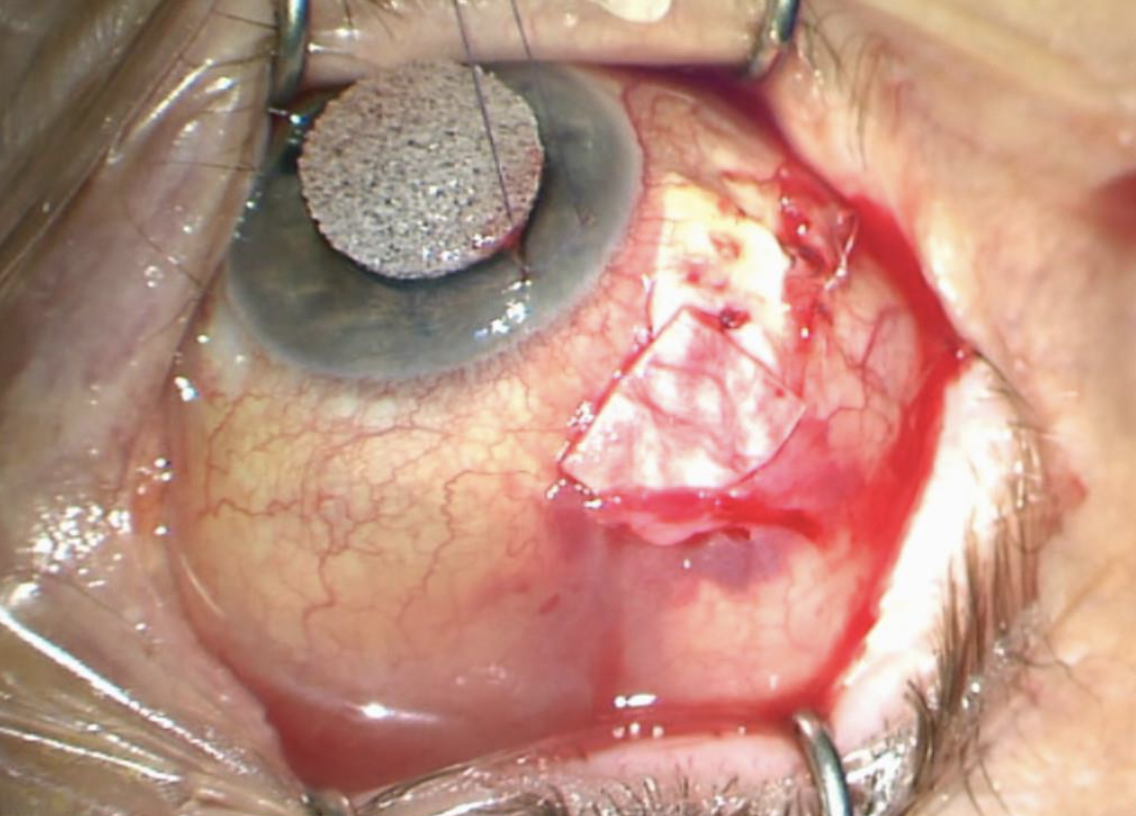 |
| Figure 3. An additional patch graft was placed but the conjunctiva couldn’t close. |
Case Example: Procedure
In this patient, Tenon’s capsule has scarred down to the underlying sclera, to the tube and to the patch graft. My goal is to try to mobilize the conjunctiva by dissecting it off Tenon’s capsule. This will create enough redundancy to enable the conjunctiva to come forward to the incision line in a tension-free way.
I start by finding the plane to separate conjunctiva off from underlying Tenon’s capsule. Using tying forceps and Wescott scissors, I perform a combination of blunt and sharp dissection (Figure 4A). Pulling the conjunctiva over the scissors (Figure 4B) ensures direct visualization so you won’t cut through the conjunctiva inadvertently and create a buttonhole.
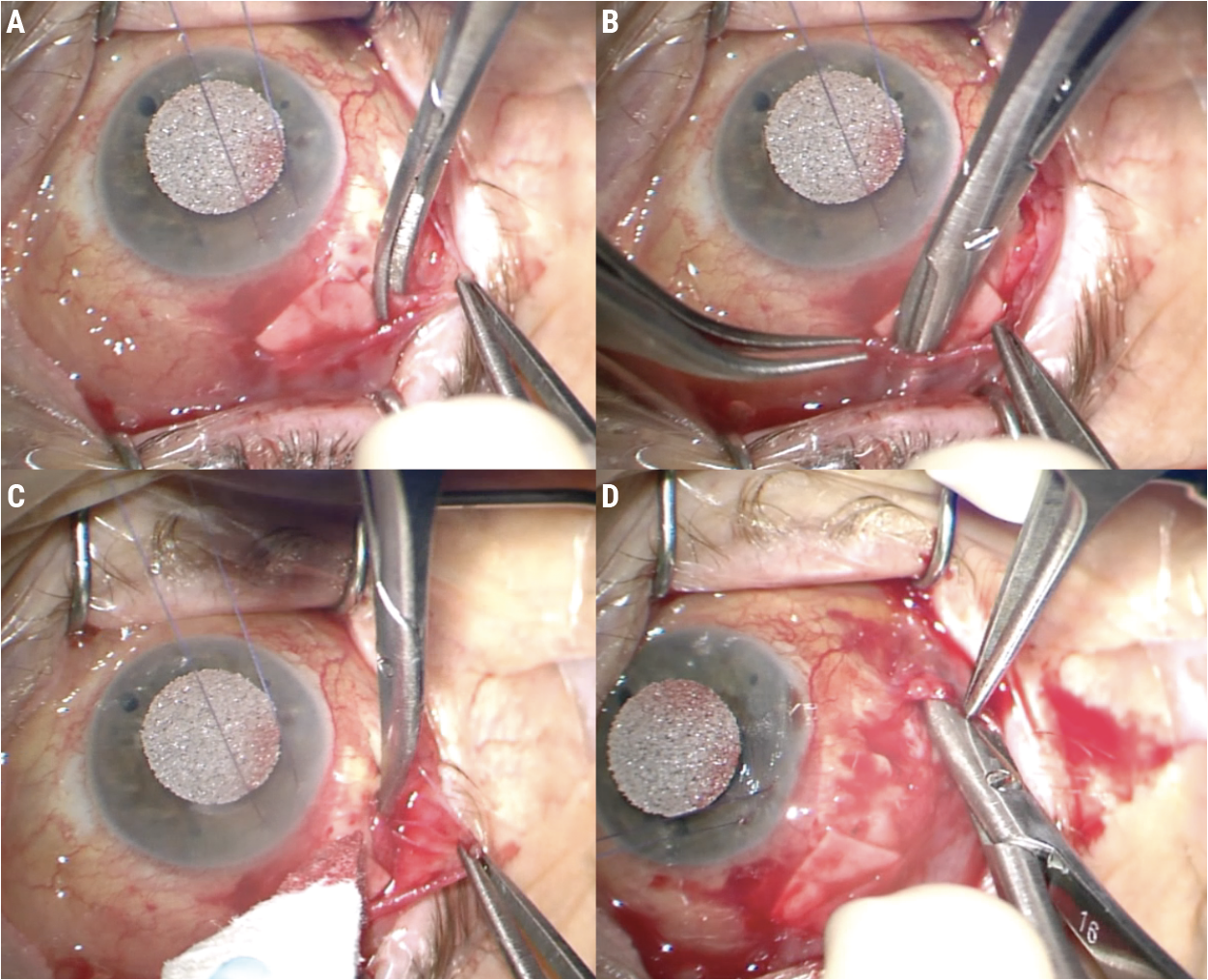 |
| Figure 4. Blunt and sharp dissection is used to separate Tenon’s capsule from the conjunctiva (A). Pulling the conjunctiva over the scissors will help avoid creating a buttonhole (B). Test the tissue’s mobility periodically (C) and dissect broadly (D). |
Be sure to test the mobility of the tissue as you work (Figures 4C-D). Dissect broadly and work until the conjunctiva comes up to the incision line in a tension-free way (Figure 5A). If you close the conjunctiva under tension, it’ll pull itself apart again.
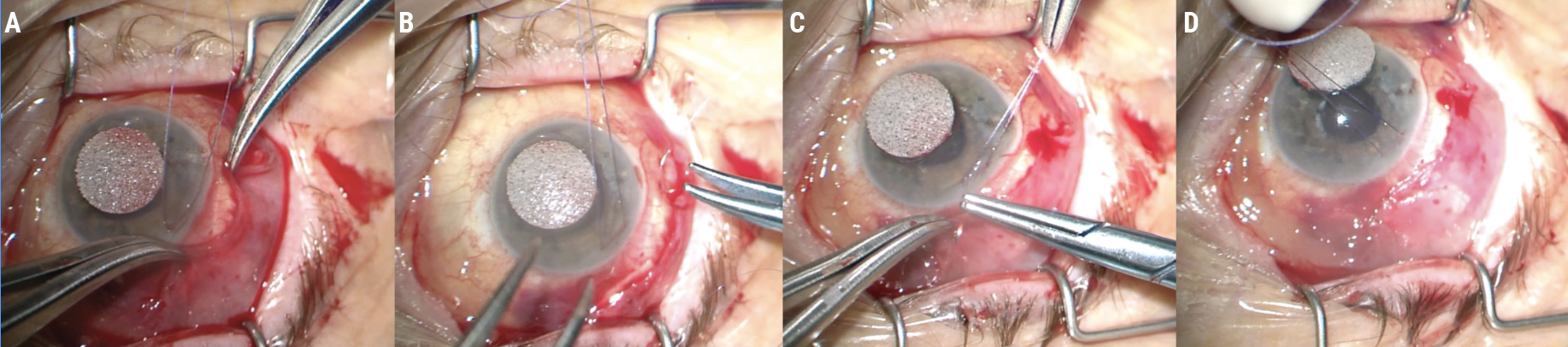 |
| Figure 5. Continue dissecting until the conjunctiva comes up to the incision line in a tension-free manner (A). Place anchoring sutures at the posterior lip of the conjunctiva to secure it to the sclera and relieve tension on the incision (B). Suture the conjunctiva in place (C). The conjunctiva should close without tension (D). |
Using a CS160-8 needle, I then place a few anchoring sutures with 9-0 Vicryl monofilament through the posterior lip of the conjunctiva to secure it anteriorly to the sclera (Figure 5B). These initial sutures help to relieve any tension hanging on the incision. Finally, I use the same suture in a running fashion to close the conjunctiva (Figures 5C-D). (See a video of this procedure below.)
At postop day 10, the patient’s visual acuity was 20/60 and her pressure was 3 mmHg with a deep anterior chamber. Conjunctival closure remained intact.
In some cases, there’s inadequate conjunctival tissue to cover the defect. In this situation, the clinician can consider circumferential relaxing incisions posteriorly in the fornix, pedicle flaps or autologous conjunctival patch grafts.6
Conjunctival Incisions
The two most commonly used conjunctival incisions for Ahmed valve placement are shown in Figure 6. The patient in the case example had their tube placed using the incision shown in red. This is a circumferential incision, usually placed 3 to 5 mm behind the limbus. The incision shown in green is a limbal peritomy with radial relaxing incisions at each end. Importantly, the incision type itself doesn’t affect dehiscence likelihood,4 but rather, the type of incision matters in terms of what happens in the event the incision splits open after surgery.
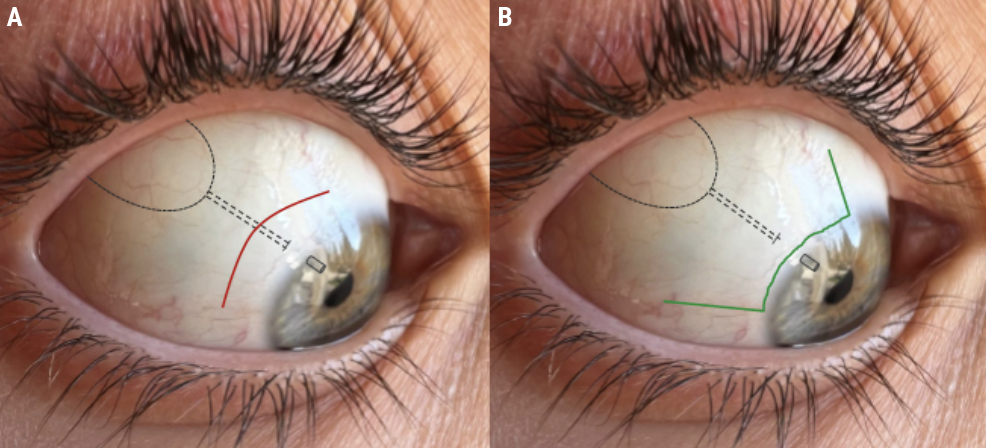 |
| Figure 6. A diagram of Ahmed glaucoma valve placement with a circumferential incision (A, red) and a limbal peritomy with radial relaxing incisions at each end (B, green). |
Accessing the plate and placing the tube using a circumferential incision involves less tissue dissection. However, if the incision splits open, this type of incision exposes the tube implant or plate beneath it (Figure 7A). On the other hand, if the radial relaxing incisions open up, there’s only bare sclera underneath them (Figure 7B). I see these open up every once in a while, and the native conjunctiva typically heals in very nicely. Oral doxycycline may be used for conservative management of conjunctival retraction without the need for the surgery.7
When placing tubes, I prefer using a limbal peritomy with radial relaxing incisions. I tunnel the tube, so it runs across the surface of the sclera (dotted line, Figures 6 and 7) and enters the anterior chamber through a scleral tunnel about 3 mm from the limbus. This ensures that if the conjunctiva retracts from the limbus a bit, it won’t expose the tube, only native sclera or sometimes the scleral graft beneath it.
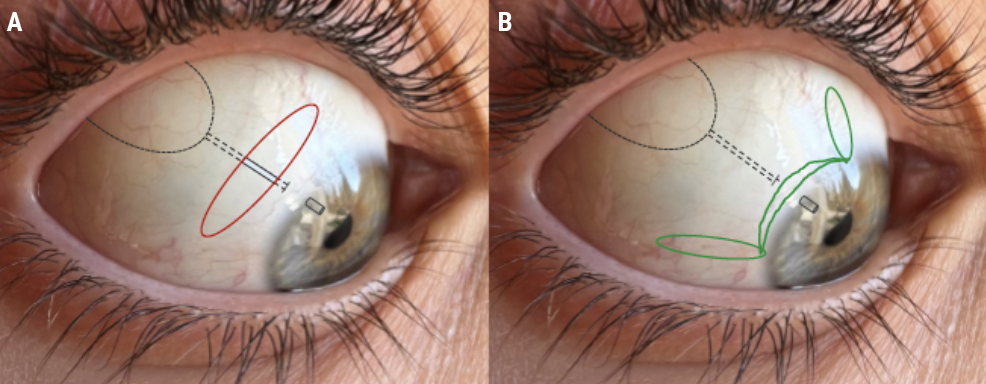 |
| Figure 7. If conjunctival dehiscence occurs with a circumferential incision (A, red), the underlying tube implant or plate may become exposed, requiring immediate repair. A limbal peritomy with relaxing incisions (B, green) won’t expose hardware since there’s only bare sclera beneath it. These dehiscences will often heal on their own. |
Pearls for Success
Conjunctival dehiscence repair often involves working with friable and/or scarred-down tissue. Here are some pearls to ensure good closure:
• Loosen the speculum and traction suture. When performing surgical maneuvers on the top part of the eye, the traction suture pulls the eye down and speculum holds the eye open. However, this puts the conjunctiva under a lot of stress and can make it very difficult to advance to the limbus. Loosening the speculum and loosening the traction suture to let the eye return to a more neutral position will make a difference when it comes to mobilizing the conjunctiva up to the limbus or incision.
• Widely mobilize the conjunctiva. Be sure to use meticulous blunt and sharp dissection when mobilizing tissue. You may need to dissect widely to get the conjunctiva to come up to the limbus. As I mentioned previously, a tension-free closure is key for repairing dehiscence. If you have to pull hard on the tissue to get the conjunctiva to come up, it’s likely to pull itself apart later.
• Avoid cautery when possible. Cautery causes tissue to shrink. For obvious reasons, this is inadvisable if you already have barely enough tissue to close the wound.
• Use monofilament suture for closure. In the case example, 8-0 Vicryl was used following the pars plana vitrectomy and tube placement. This is a braided suture, and it’s a popular choice because it’s strong and flexible. I’ve found it can be irritating to the conjunctiva, however. In Figure 3, the tissue is quite red around the sutures.
Monofilament suture, on the other hand, is a single filament as the name suggests. It’s stiffer and the suture knot tails can be more uncomfortable for the patient. However, there are advantages to this type of suture when it comes to repairing the conjunctiva. As a single filament, it’s smooth and pulls through tissue easily without cutting or abrading. Therefore, monofilament sutures are much less likely to cheese-wire through the tissue. They also seem not to inflame the conjunctiva as much as a braided suture, where the rough, braided texture can collect debris and cause inflammation. I’ve found that with monofilament suture the conjunctiva tends to be quieter.
• Use postoperative steroids conservatively. Don’t stymie the healing process. Allowing the tissue to heal and scar in place will help to avoid any future retraction or dehiscence.
In summary, if the conjunctiva falls apart, it’s important to keep the following in mind: separate the conjunctiva from Tenon’s capsule, ensure you’ve done a broad enough dissection with sufficient mobility and ensure the closure is tension free.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis.
Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
Dr. Eisengart is a glaucoma specialist at the Cleveland Clinic Cole Eye Institute in Ohio. He has no related financial disclosures.
1. Gedde SJ, Scott IU, Tabandeh H, et al. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology 2001;108:7:1323-1327.
2. Panda S, Khurana M, Vijaya L, George R, Balekudaru S. Comparison of conjunctiva-related complications between scleral and corneal patch grafts in Ahmed glaucoma valve implantation. Indian J Ophthalmol 2023;71:3:881-887.
3. Chaku M, Netland PA, Ishida K, Rhee DJ. Risk factors for tube exposure as a late complication of glaucoma drainage implant surgery. Clin Ophthalmol 2016;10:547-553.
4. Geffen N, Buys Y, Smith M, et al. Conjunctival complications related to Ahmed glaucoma valve insertion. J Glaucoma 2014;23:2:109-114.
5. Miraftabi A and Nilforushan N. Wound dehiscence and device migration after subconjunctival bevacizumab injection with Ahmed glaucoma valve implantation. J Ophthalmic Vis Res 2016;11:1:112-115.
6. Lawrence SD, Netland PA. Inadequate conjunctival coverage. In: Feldman RM, Bell NP, eds. Complications of Glaucoma Surgery. New York, NY: Oxford University Press, 2013, pp. 258-264.
7. Gupta P, Senthil S, Srirampur A, Mittal P. Role of oral doxycycline in the management of conjunctival dehiscence following glaucoma drainage device. Int Surg J 2017;4:7:2369.



