Pathophysiology of DME
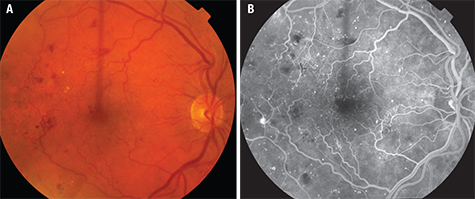
Early in DR, there are changes in the structure and cellular composition of the microvasculature. Damage to the endothelial cells that are responsible for maintaining the blood-retinal barrier (BRB) leads to increased vascular permeability. In DME, breakdown of the inner BRB allows accumulation of extracellular fluid in the macula (See Figure 3). Damage to pericytes that are essential cellular components for regulating capillary perfusion in the retina leads to altered retinal hemodynamics, including abnormal autoregulation of retinal blood flow.3 The loss of retinal pericytes occurs early in DR and correlates with microaneurysm formation.4,5 In individuals with diabetes, the capillary basement membrane thickens and increased extracellular matrix components are deposited, and these events may be contributing factors to the development of abnormal retinal hemodynamics, including the abnormal autoregulation of retinal blood flow. Many interrelated pathways are linked to the cellular damage from hyperglycemia and hypoxia affecting the BRB, including angiogenic growth factors and inflammatory cytokines. Corticosteroids modulate these pathways to exert a therapeutic effect in DME.
|
Inflammation also plays an important role in diabetic retinopathy and diabetic macular edema. Leukostasis, adhesion molecules, prostaglandin up-regulation and retinal accumulation of macrophages occur in diabetes. Retinal leukostasis, in particular, may play a key role in the pathogenesis of DR. Leukocytes possess large cell volume, high cytoplasmic rigidity, a natural tendency to adhere to the vascular endothelium and a capacity to generate toxic superoxide radicals and proteolytic enzymes.7-9 In diabetes, increased leukostasis affects retinal endothelial function, retinal perfusion, angiogenesis and vascular permeability. In patients with diabetes, leukocytes are also abnormal. They are less deformable; a higher proportion than usual are activated; and they appear to be involved in capillary nonperfusion, endothelial cell damage and vascular leakage in the retinal microcirculation. Numerous inflammatory mediators have been involved in diabetic retinopathy including tumor necrosis factor α (TNF-α), a pro-inflammatory cytokine and interleukin-6.
Pharmacotherapy of DME
Recently, intravitreal anti-VEGF agents, bevacizumab, ranubizumab and aflibercept have been used to treat DME. These three intravitreal agents bind VEGF, thereby decreasing angiogenesis and vascular permeability, causing regression of diabetic neovascularization and reduction in DME respectively. Several recent clinical trials suggest that anti-VEGF therapies are more effective than laser therapy.10 However, anti-VEGF therapy requires repeated intravitreal injection, sometimes monthly or even indefinitely. Furthermore, anti-VEGF therapy is not effective in all patients, possibly because targeting VEGF does not suppress all the inflammatory cytokines involved in DME.
Corticosteroids can be utilized in sustained-released forms to treat DME. Corticosteroids inhibit macrophages that release angiogenic growth factors and down-regulate ICAM-1, which mediates leukocyte adhesion and transmigration; they have been noted to decrease major histocompatibility complex (MHC)-II expression in the subretina where AMD-associated neovessels form.11-13 In addition to this anti-inflammatory mechanism, corticosteroids alter the composition of endothelial basal membrane by changing the local ratio of two laminin isoforms, suppressing basement membrane dissolution, and strengthening tight junctions to limit permeability and leakage that cause macular edema.11,13
Triamcinolone Acetonide
Triamcinolone acetonide has been studied in numerous clinical trials for diabetic macular edema as far back as the late 1990s.14-21 More recently, the Diabetic Retinopathy Clinical Research Network (DRCR) has studied both posterior subTenon and intravitreal TA for DME. The DRCR protocol I represented a pivotal clinical trial assessing three different treatment schemes: intravitreal 0.5 mg ranibizumab plus prompt or deferred focal/grid laser; or 4 mg intravitreal TA combined with focal/grid laser compared with focal/grid laser alone.22
|
Intravitreal TA has a half-life of 18.6 days and may persist at levels sufficient to exert clinical effect for up to three months.23 Nevertheless, repeat injections are required, which can increase the risk of cataract and glaucoma. Instead of intermittent bolus therapy, it is thought that sustained release of a lower-dose corticosteroid may lead to greater efficacy with fewer complications of cataract or glaucoma. This has led to the development of the DEX and FA implants.
Dexamethasone Implant
In June 2009, the Food and Drug Administration approved a 0.7-mg DEX implant contained in a solid bioerodable polymer for the treatment of macular edema following retinal vein occlusion. It can exert a clinical effect for three to six months. In September 2010, the 0.7-mg implant was approved for treatment of non-infectious intermediate and posterior uveitis. In June 2014, it was approved for the use in DME in pseudophakic patients or those phakic patients scheduled for cataract surgery. In September 2014, approval was expanded for the use in general DME patients, both pseudophakic and phakic.
In a clinical trial known as the MEAD Study, 1,048 subjects with DME with best-corrected visual acuity 34 letters (20/200) to 68 letters (20/50), and center macula subfield thickness >300 µm were randomized in a 1:1:1 ratio to DEX implant 0.7 mg, DEX implant 0.35 mg or sham procedure and followed for three years.24 Subjects were eligible for retreatment every six months based on predefined OCT criteria. The percentage of patients with ≥15-letter improvement in BCVA from baseline at study end was greater with DEX implant 0.7 mg (22.2 percent) and DEX implant 0.35 mg (18.4 percent) than sham (12 percent; p≤ 0.018). Mean average reduction in CRT from baseline was greater with DEX implant 0.7 mg (-111.6 μm) and DEX implant 0.35 mg (-107.9 μm) than sham (-41.9 μm; p< 0.001).
The DEX implant may be particularly well-suited for the treatment of DME in vitrectomized eyes; these eyes may more rapidly clear intermittently administered intravitreally injected drugs compared to nonvitrectomized eyes. A clinical trial known as the CHAMPLAIN study evaluated 55 patients with treatment-resistant DME and a history of previous pars plana vitrectomy. The study eyes received a single 0.7-mg DEX intravitreal implant and were followed for 26 weeks. These eyes showed statistically and clinically significant improvements in both visual acuity and vascular leakage from DME at 26 weeks. At week eight, 30.4 percent of patients had gained ≥10 letters in BCVA.25
Fluocinolone Acetonide Implant
The FA intravitreal implant is administered using a 25-ga. inserter, and it leads to sustained drug release for up to 36 months. Unlike the DEX implant, it is not bioerodable. In 2005, an FA intravitreal implant containing 0.59 mg FA was approved in the United States for the treatment of non-infectious uveitis. In a clinical trial known as the FAMOUS study, 37 patients with persistent DME despite prior focal/grid laser therapy were randomized 1:1 to receive an experimental intravitreal injection of a 0.2- or a 0.5-μg/day insert.26 After administration of a 0.2-μg/day insert, the mean change from baseline in BCVA was 5.1, 2.7 and 1.3 letters at months three, six and 12, respectively. The mean change from baseline after administration of a 0.5 μg/day-insert was 7.5, 6.9 and 5.7 letters at months three, six and 12, respectively. Aqueous humor sampling revealed sustained intraocular release of FA for greater than one year.
The Fluocinolone Acetonide for Diabetic Macular Edema (FAME) studies evaluated 953 eyes of patients with persistent DME after ≥one laser therapy treatments, randomized 1:2:2 for sham injection (n=185), low-dose FA insert (0.2 μg/day, n=375) or high-dose FA insert (0.5 μg/day, n=393).27,28 At 36 months, 27.8 percent (high dose) and 28.7 percent (low dose) of implant-treated eyes versus 18.9 percent of sham eyes demonstrated an improvement of 15 or more letters (p=0.018). A subgroup analysis showed particular benefit among patients with DME for three or more years. Corticosteroid-related side effects were noted; up to 8.1 percent required incisional glaucoma surgery, and cataracts progressed in nearly all phakic eyes.
In September 2014, the FDA approved FA implant containing 0.19 mg fluocinolone for DME in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure. However, this previous course of corticosteroids was not specified. Clinicians could conceivably trial a topical corticosteroid, intravitreal bolus therapy with triamcinolone or DEX implant.
Complications
The exact mechanism of corticosteroid-induced secondary intraocular pressure rise is not known; however, one established contributory factor is increased outflow resistance within the trabecular meshwork.29,30 In the FAME study, there was a greater need for surgical glaucoma intervention at the three-year point in patients receiving the FA injection group; 2.5 percent of the high-dose group, 1.3 percent of the low-dose group and 0 percent of the sham injection group required laser trabeculoplasty. Incisional glaucoma surgery was needed in 8.1 percent of the high-dose group, 4.8 percent of the low-dose group and 0.5 percent of the sham injection group.27 In the MEAD study, ocular hypertension was usually controlled with medication or no therapy; only two patients (0.6 percent) in the DEX implant 0.7-mg group and one (0.3 percent) in the DEX implant 0.35-mg group required trabeculectomy.24
|
Ocular corticosteroid therapy is known to cause secondary cataract formation, a complication also associated with administration of systemic corticosteroids. The FAME study reported that cataract development rates were higher in those patients receiving FA inserts; they reported that 42.7 percent of the low-dose group, 51.7 percent of the high-dose group, and 9.7 percent of the sham injection group developed cataracts. These numbers represent 81.7 percent, 88.7 percent and 50.7 percent respectively of the patients in each group with phakic eyes at the start of the study.27 In the MEAD study, rates of cataract formation in phakic eyes were 67.9 percent, 64.1 percent and 20.4 percent in the DEX implant 0.7 mg, DEX implant 0.35 mg and sham groups, respectively.24
In summary, although anti-VEGF therapy is becoming the treatment of choice for center-involved DME, the recently approved sustained-release low-dose DEX implant and FA implant add greatly to the treatment options. In particular, these implants will limit frequent intravitreal injection, often required with intravitreal anti-VEGF therapy. Corticosteroid implants may also limit the cost of repeated treatment with expensive anti-VEGF therapies such as ranibizumab or aflibercept and may minimize the risk of endophthalmitis, given the lower number of injections. While the FA implant lasts much longer than the DEX implant, potentially decreasing the visit and treatment burden on patients and their families, the FA implant appears to have a greater risk of ocular hypertension and cataract. However, these modalities have not been directly compared in a clinical trial.
There is insufficient evidence to draw more elaborate conclusions, especially to determine if multiple injections with the DEX implant lead to the same risks as the longer-lasting FA implant. As noted above, the FA implant’s approval requires a prior treatment with a course of corticosteroids to rule out a clinically significant rise in intraocular pressure. However, this prior course of corticosteroids could conceivably be a topical corticosteroid, intravitreal bolus therapy with triamcinolone, or DEX implant.
There are no large randomized prospective clinical trials comparing sustained-release corticosteroid therapy to antiVEGF therapy as first-line therapy in center-involved DME, but DEX and FA implants could become early treatment for pseudophakic patients. For non-center-involved DME, laser treatment could remain first-line treatment, since the risks of laser photocoagulation are minimal in these cases, compared to the risks, discomfort and expense of intravitreal therapies. DEX or FA implants might be especially attractive as early therapies for center-involved DME in eyes that have undergone vitrectomy, since it is thought that anti-VEGF agents have shorter half-life, and presumable less efficacy in these cases.
For center-involved DME that is persistent despite periodic anti-VEGF therapy, the durable action of corticosteroid implants, especially the FA implant, facilitates combination therapy. In the future, patients could receive these implants as foundational therapy, and then receive additional treatment with laser or intravitreal anti-VEGF agents as combination therapy, which may conceivably provide some synergistic benefit. FA may be particularly attractive for this use in pseudophakic patients without significant risk of glaucoma, given its long duration of action.
Finally, corticosteroids implants may have a special role in the treatment of chronic DME. A recent study compared the efficacy of the FA implant in chronic (≥three years) versus non-chronic (<three years) DME in a preplanned subgroup analysis of the FAME study.32 At month 36, the difference between FA implant and sham control in the percentage of subjects who gained 15 letters or more was significantly greater in 536 chronic DME subjects (34 percent vs. sham, 13.4 percent; p<0.001), compared to the 416 subjects with non-chronic DME (22.3 percent vs. sham, 27.8 percent; p=0.275). The differences could not be explained by baseline ocular characteristics, changes in anatomic features or differences in re-treatment or ancillary therapies. The authors speculate that early DME is driven primarily by VEGF, while chronic DME may driven more by inflammatory cytokines in addition to anatomic changes. The authors conclude that the FA implant may be an option for patients who do not respond to other therapy. This report may also partially account for the clinical observations of beneficial effect using the DEX implant when anti-VEGF agents have minimal effect. REVIEW
Dr. Ciulla is on the Retina Service at Midwest Eye Institute, 200 W. 103rd St. Indianapolis, IN 46290. Contact him at (317) 817 1822 or e-mail thomasciulla@gmail.com.
1. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995;102:7-16.
2. Ferris FL, 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 1984;28 Suppl:452-61.
3. Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand 2002;80(5):468-77.
4. Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003;26(9):2653-64.
5. Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: Physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmologica. 2010;88(3):279-91.
6. Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 2013;120:106-14.
7. Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin Ophthalmol 1999;14(4):233-9.
8. Miyamoto K, Hiroshiba N, Tsujikawa A, Ogura Y. In vivo demonstration of increased leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci 1998;39(11):2190-4.
9. Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci 2000;41(5):1153-8.
10. Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Therapeutic advances in endocrinology and metabolism. 2013;4(6):151-69.
11. Tokida Y, Aratani Y, Morita A, Kitagawa Y. Production of two variant laminin forms by endothelial cells and shift of their relative levels by angiostatic steroids. The J Biol Chem 1990;265(30):18123-9.
12. Ingber DE, Madri JA, Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: Induction of capillary basement membrane dissolution. Endocrinology 1986;119(4):1768-75.
13. Stokes CL, Weisz PB, Williams SK, Lauffenburger DA. Inhibition of microvascular endothelial cell migration by beta-cyclodextrin tetradecasulfate and hydrocortisone. Microvasc Res 1990;40(2):279-84.
14. Audren F, Erginay A, Haouchine B, Benosman R, Conrath J, Bergmann JF, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular oedema: 6-month results of a prospective controlled trial. Acta Ophthalmol Scand 2006;84(5):624-30.
15. Audren F, Lecleire-Collet A, Erginay A, Haouchine B, Benosman R, Bergmann JF, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: Phase 2 trial comparing 4 mg vs 2 mg. Am J Ophthalmol 2006;142:794-99.
16. Jonas JB, Kamppeter BA, Harder B, Vossmerbaeumer U, Sauder G, Spandau UH. Intravitreal triamcinolone acetonide for diabetic macular edema: A prospective, randomized study. J Ocul Pharmacol Ther 2006;22(3):200-7.
17. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 2003;121:57-61.
18. Jonas JB, Sofker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol 2001;132:425-7.
19. Jonas JB, Spandau UH, Kamppeter BA, Vossmerbaeumer U, Harder B, Sauder G. Repeated intravitreal high-dosage injections of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 2006;113:800-4.
20. Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002;109:920-7.
21. Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: Preliminary results of a prospective controlled trial. Ophthalmology 2004;111:218-24; discussion 24-5.
22. Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, 3rd, Friedman SM, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118:609-14.
23. Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003;110:681-6.
24. Boyer DS, Yoon YH, Belfort R, Jr., Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904-14.
25. Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina 2011;31:915-23.
26. Campochiaro PA, Hafiz G, Shah SM, Bloom S, Brown DM, Busquets M, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology 2010;117:1393-9 e3.
27. Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012;119:2125-32.
28. Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011;118:626-35 e2.
29. Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res 2009;88(4):769-75.
30. Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: Towards a better understanding of glaucoma. Prog Retin Eye Res 1999;18(5):629-67.
31. Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, et al. Intraocular pressure monitoring post intravitreal steroids: A systematic review. Surv Ophthalmo 2013;58(4):291-310.
32. Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, et al. Sustained delivery fluocinolone acetonide vitreous implants: Long-term benefit in patients with chronic diabetic macular edema. Ophthalmology 2014;121:1892-903.