As both a practicing ophthalmologist and a dry-eye sufferer, I have a unique perspective on this "treatment gap." Through this article I hope to increase awareness of dry eye as a common condition, and encourage my fellow ophthalmologists to uncompromisingly diagnosis and treat it.
Etiology and Pathogenesis
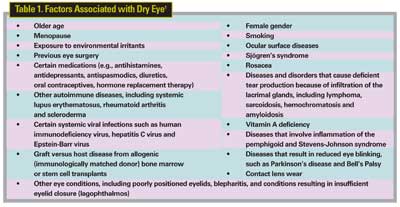
Dry eye results from reduced tear production, excessive tear evaporation or abnormal tear composition. It may occur sporadically, for example, with exposure to dry or windy environments; or it can be a chronic condition referred to as dry-eye syndrome, or keratoconjunctivitis sicca. The condition is associated with a wide variety of individual, environmental, and disease-related factors (See Table 1).
Dry eye due to deficient tear production is often designated as "Sjögren's" or "non-Sjögren's," with the latter precipitated by exposure to allergens and irritants, or certain systemic medications. In addition to factors listed in Table 1, dry eye can be associated with other conditions such as diabetes and androgen suppression in men being treated for prostate cancer.
It is now recognized that inflammation is an underlying cause of both Sjögren's and non-Sjögren's tear-deficient dry eye. In the normal state, the proteins and other antigenic substances secreted by epithelial cells and lacrimal glands are recognized by local macrophages, which present them to effector T cells, initiating an immune cascade and local inflammatory response. This inflammation is kept under control, however, by regulatory T cells.2,3 This balance can be tipped toward excessive inflammation by the various factors listed in Table 1. For example, a decrease in testosterone associated with aging or menopause results in a decrease in regulatory T cell activity.4
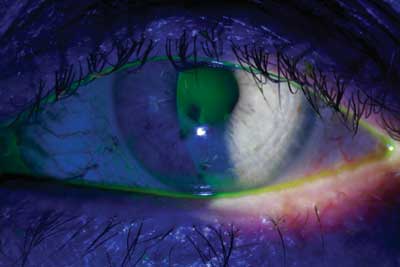
Tear breakup time is one of several tests available to diagnose dry eye.
Once excessive inflammation takes hold, it can lead to a vicious cycle: effector T cells secrete more pro-inflammatory cytokines into the tear film, recruiting more effector T cells and stimulating still greater inflammation, causing changes in tear production, quantity and/or quality.5 Over time, this can damage the ocular surface and the tear-secreting glands, making dry eye worse. Thus, inflammation is both a cause and a result of damage to the ocular surface.2,3,6,7
This theory has major implications for treatment.2,3 Specifically, Restasis (cyclosporine ophthalmic emulsion 0.05%, Allergan) suppresses inflammation due to effector T cells, helping increase natural tear production. Currently, this is the only prescription therapy for patients with dry eye due to deficient tear production resulting from ocular inflammation. Other medications are currently in Phase II and III studies, or awaiting approval by the Food and Drug Administration.
A Common Condition
The relatively few reported estimates of dry-eye prevalence vary because of differences in data-gathering methods and diagnostic criteria. Moreover, reports probably underestimate prevalence because many people who have dry-eye symptoms don't discuss them with their health-care provider. One report estimated approximately 20 million Americans suffer from dry eye.8 A population-based study of 3,722 participants in Beaver Dam, Wis. found dry eye in 14.4 percent of adults 48 to 91 years old, including 8.4 percent of those younger than 60 and 19 percent of those older than 80.9
Menopausal and post-menopausal women are especially susceptible to dry eye. Dry eye is twice as common in women compared to men, affecting more than 3.2 million American women over age 50.10
There is relatively little data on the direct and indirect costs of dry eye. Obviously it can significantly affect quality of life, increasing problems with reading, professional work, outdoor activities, computer use and driving.11,12 One study found dry eye resulted in an average of 184 days of reduced productivity in a year, or a loss of $5,362 per person. (Kozma CM, et al. IOVS 2000;41:ARVO Abstract 4933)
Not surprisingly, better diagnosis and management of dry eye can save costs. For example, better management reduces the risk of bacterial keratitis, which can require corneal transplantation, a $20,000 procedure.13 A study showed that therapy with Restasis resulted in symptom improvement in 60 percent of patients and 55 percent fewer medication orders, including those used for dry eye.14 Finally, better management also would reduce indirect costs of dry eye, such as lost or decreased work productivity.13
Underdiagnosed, Undertreated
While dry eye accounts for nearly one-fourth of all ophthalmological office visits,15,16 the disease remains under-diagnosed. Older women with the condition may not realize it's treatable, and so don't discuss their symptoms with their physician or ophthalmologist. And despite the higher prevalence in this population, many physicians and ophthalmologists aren't proactive in querying for and investigating symptoms and signs.
Closing the Gap
Since many women do not spontaneously discuss their dry-eye symptoms with their physicians, the responsibility to recognize and discuss dry eye symptoms falls to the physician. These symptoms can be similar to those due to other causes, such as allergies, eye strain, contact lens intolerance, and other diseases affecting the ocular surface. Even in cases of dry eye, symptoms may not correlate well with clinical signs. Therefore, it's generally recommended that a diagnosis of dry eye should be based on multiple assessments, including patient history, physical examination and specific diagnostic tests.
A patient history is important not only to establish a diagnosis of dry eye, but also to identify possible causes. Because a wide variety of factors can contribute to dry eye, the history needs to cover a lot of ground (See Table 2). The history should capture the patient's specific symptoms, as they can vary greatly (See Table 3).

A number of standard patient history questionnaires are available. The McMonnies questionnaire has been widely used for the last 20 years. More recent tools include the Ocular Surface Disease Index (OSDI), the Dry Eye Questionnaire (DEQ), and the National Eye Institute Visual Function Questionnaire (NEI VFQ-25). These instruments assess symptoms of discomfort, use of medications, change in activities and, in the case of the OSDI and NEI VFQ-25, changes in visual function and environmental stimuli.
The physical examination includes a visual acuity measurement, an external examination (especially focusing on the patient's skin, eyelids, tear glands, eye structure, cranial nerve function, face and hands) and slit-lamp biomicroscopy. While the physical examination often reveals clinical signs that can cause or contribute to dry eye, it may not always detect inflammation, an underlying etiology.
Several diagnostic tests are useful to confirm a dry eye diagnosis, evaluate disease severity and, increasingly, to assess therapeutic response, including: tear breakup time (TBUT); tear film meniscus height; and ocular surface staining with rose bengal, fluorescein or lissamine green dye.17 Fluorescein is used to stain the cornea and the areas of epithelial drop-out will show with this dye. Rose bengal and lissamine green are vital dyes that are taken up by devitalized conjunctival or corneal cells. Positive staining of the conjunctiva with rose bengal or lissamine green is consistent with a diagnosis of dry eye.
• Schirmer Testing is useful for diagnosing aqueous tear deficiency, but gives variable results, and so is not recommended as the sole criterion for diagnosing dry eye.
• More involved and invasive procedures—including tear film osmolarity, lactoferrin, lysozyme immunoglobulin and albumin, and impression cytology—may be useful in quantifying dry eye for research purposes, but are not practical for routine clinical diagnosis.
There are a number of diagnostic classification schemes for dry eye, including one developed in 1995 by the National Eye Institute18 and in 2003 by the American Academy of Ophthalmology,1 which are reviewed annually and updated at least every five years. In 2004, an International Task Force (ITF) of dry-eye specialists created guidelines for diagnosing and treating dry eye designed to improve patient outcomes and increase practice efficiencies.
The ITF decided that the term "dry eye" does not adequately describe the diverse underlying causes, symptoms and clinical signs of this group of disorders, so it recommended the term "dysfunctional tear syndrome" (DTS), and the following diagnostic classification:19
1. Clinical category
• DTS with no lid margin disease (inflammation of the eyelid)
• DTS with lid margin disease
• DTS with altered tear film distribution/clearance
2. Inflammation
• Apparent
• Not apparent
3. Severity level based on symptoms, clinical signs and diagnostic tests. (Note: three symptoms are considered—ocular discomfort, ocular fatigue and visual disturbances—and each is ranked on a scale of zero [none] to four [extremely severe]).
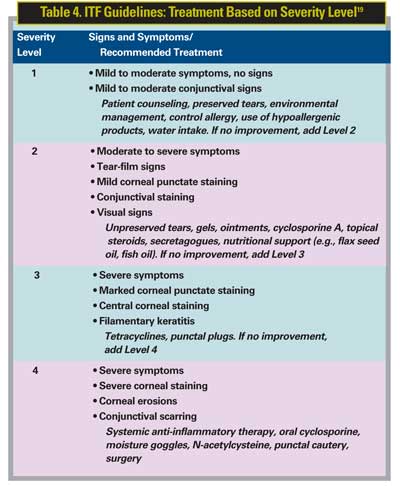
• Level 1. Mild to moderate symptoms; mild to moderate conjunctival signs; no staining.
• Level 2. Moderate to severe symptoms; tear film signs; visual signs; mild corneal punctate staining; conjunctival staining.
• Level 3. Severe symptoms; corneal filamentary keratitis; marked corneal punctate staining; central corneal staining.
• Level 4. Extremely severe symptoms/altered lifestyle; conjunctival scarring; corneal erosions; and severe corneal staining.
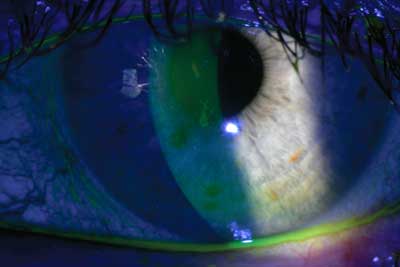
Fluorescein stain on the cornea will reveal areas of epithelial drop-out.
Treatment
The goals of dry eye treatment include: reducing or alleviating signs and symptoms; maintaining visual function; reducing or preventing structural damage; and preventing progression.
Dry-eye diagnostic and management guidelines published by the AAO recommend education, environmental control and artificial tears as the beginning of the treatment continuum, with medications and surgery recommended for progressively more severe cases. Unfortunately, these guidelines are not in widespread use.1 A survey found that 65 percent of specialists use no dry eye treatment guidelines, but would be interested in guidelines that were current, applicable and used a practical treatment algorithm.1

To address this need, the ITF developed treatment guidelines based on symptom frequency and quality of life impact. Overall, the ITF made three main recommendations related to treatment:19
• The primary determinant of therapeutic strategy should be the severity of symptoms and signs.
• It is important to observe a patient's response and change therapy if necessary.
• Anti-inflammatory therapy should be used in level two severity or worse, regardless of the level of clinically apparent inflammation.
The ITF recommends that if symptoms do not improve, treatment components at the next level of dry-eye severity should be added (See Table 4).
The ITF guidelines also are not in widespread use and not all dry eye specialists follow the guidelines as written.
Major Treatment Components
In addition to environmental approaches, there are three main dry-eye treatment options: artificial tears; prescription therapies and surgery.
Artificial tears and eye drops primarily provide symptomatic relief and do not address the underlying cause of dry-eye disease; however, they may be adequate for treating mild or episodic dry eye. Further, as shown in the ITF Guidelines, they are a "foundation" therapy for all levels of severity; therefore, they are recommended for severity level one, and remain as a treatment component for other severity levels as additional therapies are added.
Packaged in large multidose bottles, preserved artificial tears are convenient, but the preservatives themselves can cause corneal irritation, especially with daily use. Some brands use milder preservatives that break down when applied to the eye. There are also preservative-free artificial tears for patients who may experience irritation with preserved artificial tears. If a patient requires artificial tears more than three to four times per day, preservative-free tears should be recommended.
Many people with dry eye use "redness-reducing" eye drops to relieve irritation. Because they work through vasoconstriction and contain preservatives, however, they can cause ocular surface damage and long-term discomfort.
As a general rule, if patients use artificial tears three or more times a day for dry-eye symptoms, they should see an ophthalmologist about additional treatments.
• Prescription Therapies. Prescription medications are recommended for patients with severity level two or greater. The main prescription therapy for dry eye due to deficient tear production due to inflammation is Restasis. In four multicenter, randomized, controlled clinical studies of approximately 1,200 patients with moderate-to-severe dry eye, this therapy significantly increased Schirmer wetting at six months.20
The systemic cholinergic agonists pilocarpine and cevimeline have been used to treat patients with dry-eye severity level three or greater, especially those with Sjögren's syndrome, though these medications appear to be more effective at treating dry mouth associated with that disorder. Topical corticosteroids have been reported to reduce eye irritation and improve the tear film, but long-term use of this drug class may be associated with severe side effects including ocular hypertension, glaucoma, cataract formation and infection. A one-to-two-week burst/taper of topical steroid in conjunction with Restasis may be considered in more severe patients. Some patients also benefit from topical or systemic antibiotic therapy such as tetracycline or erythromycin, which can reduce inflammation and improve lipid production, particularly in evaporative dry eye.21
Patients with dry-eye severity level four are prone to developing mucous plaques on the corneal surface, which can collect external dust and bacteria and cause pain. N-acetylcysteine eye drops can help dissolve mucous plaques. A number of other medications are currently under investigation, including topical hormone therapy, P2Y2 agonists, tetracyclines and retinoic acid.21
• Surgery. Surgery—including punctal occlusion or plugs, cauterization of tear ducts, or, in especially intractable cases, tarsorrhaphy—is performed in patients with symptomatic, moderate-to-severe disease for whom medical treatments do not provide adequate relief. I prefer to start Restasis two to three months prior to placement of punctal plugs, in order to improve tear quality.
Follow-up and Compliance
It is essential to follow up with patients to assess their response to therapy and the need to adjust treatment, monitor for structural ocular damage, and provide reassurance.
Poor compliance with therapy has been a common problem because, while artificial tears have a role in dry eye therapy, they do not treat the underlying cause. As a result, many patients have recurring symptoms, became discouraged, and do not follow up with their treatment plans or their eye-care professionals. The availability of new and more effective treatment options increases the likelihood that patients will stay with their treatment, but good compliance is still not guaranteed. Patients need to be educated about the nature of dry eye and understand that it requires long-term management, including modification of possible aggravating environmental and lifestyle factors. Physicians also need to help patients set realistic treatment expectations at each level of care.
Dr. Barber is is a professor of ophthalmology at the University of Arkansas for Medical Sciences, in Little Rock.
1. American Academy of Ophthalmology Cornea/External Disease Panel. Dry Eye Syndrome Preferred Practice Pattern. American Academy of Ophthalmology, San Francisco, Calif., 2003.
2. Wilson SE. Inflammation: A unifying theory for the origin of dry eye syndrome. P&T Digest 2003 (Suppl): 14-19.
3. Smith RE. The tear film complex: Pathogenesis and emerging therapies for dry eyes. Cornea 2005; 24: 1-7.
4. Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, et al. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? IOVS 1999; 40:1261-1265.
5. Pflugfelder SP, Beuerman RW, Stern ME. Dry Eye and Ocular Surface Disorders. New York, NY: Marcel Dekker; 2004.
6. Baudouin C. The pathology of dry eye. Surv Ophthalmol 2001;45 Suppl 2:S211-20.
7. Stern ME, Beuerman RW, Fox RI, et al. The Pathology of Dry Eye: The interaction between the ocular surface and lacrimal glands. Cornea 1998;17:584-589.
8. Report on the Global Dry Eye Market. St. Louis, Mo. Market Scope, July 2004.
9. Moss SE, Klein R, Klein BEK. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000;118:1264-1268.
10. Schaumberg D, Sullivan D, Buring J, Dana R. Prevalence of dry eye syndrome among U.S. women. Am J Ophthalmol 2003;136:318-326.
11. Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol 2002; 506:989-998.
12. Nelson JD,Helms H, Fiscella R, Southwell Y, Hirsch JD. A new look at dry eye disease and its treatment. Adv Ther 2000;17(2):84–93.
13. Sheppard JD. Dry eye moves beyond palliative therapy. P&T Digest 2003 (Suppl):6-8.
14. Cross WE, Lay LF, Walt JG, Kozma CM. Clinical and economic implications of topical cyclosporine A for the treatment of dry eye. Manag Care Interface 2002;15:44-49.
15. Hikichi T, Yoshida A, Fukui Y, et. al. Prevalence of dry eye in Japanese eye centers. Graefe's Arch Clin Exp Ophthalmol 1995;233:555-558.
16. Doughty MJ, Fonn D, Richter D, et. al. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patient presenting to optometric practices across Canada. Optom Vis Sci 1997;74:624-631.
17. EyeMDLink.com. Dry eye testing. Available at: http://www.eyemdlink.com/Test.asp?TestID=10. Accessed 2/16/06.
18. Lemp MA. Report on the National Eye Institute/Industry workship on clinical trials in dry eyes. CLAO J 1995;21:221-231.
19. Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, and the Dysfunctional Tear Syndrome Study Group. Dysfunctional Tear Syndrome: A Delphi Approach to Treatment Recommendations. Cornea 2006 (In Press).
20. Restasis prescribing information. Allergan, Irvine Calif. February 2004. Available at: http://www.restasisprofessional.com/_prescribe/prescribe_home.htm. Accessed 2/16/06.
21. Perry HD, Donnenfeld ED. Medications for dry eye syndrome: A drug-therapy review. P&T Digest 2003 (Suppl):26-32.



