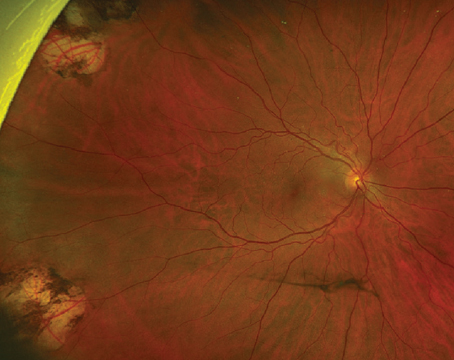
The vitreous has long been recognized as an integral factor in a wide spectrum of retinal diseases including retinal detachment, macular hole formation, vitreomacular traction syndromes, diabetic retinopathy, proliferative vitreoretinopathy and most recently, neovascular age-related macular degeneration.1-3 The pathogenic mechanisms by which the vitreous affects the retina are not completely understood, but include abnormalities in vitreoretinal adhesion manifesting in tractional phenomena, vitreous opacification and direct effects on retinal oxygenation and growth factor and cytotoxine production and distribution within the eye (See Figure 1). Currently, the pathologic manifestations of vitreopathies are managed primarily with vitrectomy (See Figure 2). However, recent developments suggest a new era of manipulation of the vitreous is imminent. Jerry Sebag, MD, coined the term "pharmacologic vitreolysis" to describe the therapeutic manipulation of molecular structure of the vitreous to achieve posterior vitreous detachment and vitreous liquefaction.4 More recently, Michael Trese, MD, has used the term "vitreodynamics" in recognition of changes in vitreous oxygenation and growth factor levels that result from pharmacologically induced changes in vitreous molecular structure.5 The majority of developmental work performed in pharmacologic vitreolysis and vitreodynamics involves enzymes including hyaluronidase, chondroitinase, tissue plasminogen activator, dispase, urokinase and plasmin.6-11 This review will discuss the current state of enzymatic vitreolysis.
Vitreous Molecular Structure
The vitreous is a unique extracellular matrix, which is ideal for pharmacologic manipulation because of its high water content and relatively few molecular and cellular components, which allows for rapid diffusion of injected agents. The predominant macromolecular components of the vitreous are hyaluronan and collagens. Hyaluronan is a disaccharide glycosaminoglycan consisting of glucuronic acid and N-acetyl glycosamine. Hyaluronan exists as a highly hydrated series of coiled, long molecular chains arranged in a linear helix.2,12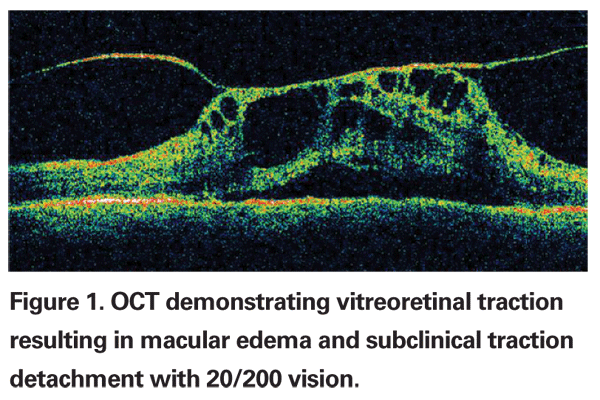
Vitreous collagens are non-branching fibrils 7 to 28 nm in diameter consisting of covalently linked collagen molecules of several types. Analysis of vitreous reveals collagen type II, a hybrid of type V and type XI and type IX distributed in a ratio of 75:10:15, respectively. Although the majority of collagen is type II, types V/XI and IX facilitate stability of the vitreous gel. Type-IX collagen promotes cohesion between the fibrils and hyaluronan. The fibrils are structured with type V/XI at the core which is surrounded by type II with type IX on the fibril surface. The assembly of these collagens enhances fibril stability and strength. It is the interaction between the collagen fibrils and hyaluronan that creates the unique optical and mechanical properties at the vitreous (See Figure 3). The large domains of hyaluronan stabilize and disperse the collagen fibrils, thereby minimizing light scattering. The viscoelastic properties of vitreous result from complex molecular interactions between the collagen fibrils and hyaluronan as well as other glycosoaminoglycans such as chondroitin 6-sulfate. These glycosaminoglycans aggregate together via hydrophobic and hydrogen bonding to create a three-dimensional honeycomb-like structure. This ordered aggregation of glycosaminoglycans is fragile, however, and this fragility may be exploited by pharmacologic agents.2,13
Many retinal diseases involve an anomalous vitreoretinal interface. The mechanical relationship between the vitreous and the retina is mediated by the posterior vitreous cortex and the internal limiting membrane. The posterior vitreous cortex consists of densely packed collagen fibrils arranged tangentially upon the ILM (See Figure 4). The adhesion of the posterior vitreous cortex is a dynamic process with topographic and age-related variations. There are no apparent gross anatomic correlates to explain the occurrence of vitreoretinal adhesion, which is therefore believed to be mediated by a molecular glue consisting of glycoproteins such as fibronectin, laminin and chondroitin, as well as integrins and other glycoconjugates. This molecular glue is an important target in pharmacologic vitreolysis.2,12
As with other extracellular matrixes, the vitreous contains matrix metalloproteinases, which are regulated by tissue inhibitors of MMP (TIMP). MMP and TIMP play both a physiologic and pathologic role in the vitreous. These enzymes contribute to the proteolytic state that clears the vitreous of potential opacification and maintains optical clarity. In disease states, MMP have been implicated in proliferative vitreoretinopathy, retinopathy of prematurity and diabetic retinopathy.14
The above understanding of vitreous molecular structure is the basis for current attempts at pharmacologic vitreolysis. Although multiple enzymes and other agents have been examined in animal models, only hyaluronidase, plasmin and microplasmin have had significant clinical experience reported.
Hyaluronidase
Hyaluronidase is the only agent for enzymatic vitreolysis which has been evaluated in Phase III clinical trials.15,16 Hyaluronidase cleaves the glycosidic bonds of hyaluronan as well as other mucopolysaccharides resulting in dissolution of the hyaluronan and collagen complex and subsequent vitreous liquefaction. There is no evidence to support a direct effect upon the vitreoretinal interface by hyaluronidase.5,17 Vitreous liquefaction increases the diffusion rate of erythrocytes and phagocytes through the vitreous thereby facilitating clearance of vitreous hemorrhage. A preservative-free, highly purified ovine hyaluronidase formulation (Vitrase, Ista Pharmaceuticals) was studied in two large Phase III clinical trials for the management of vitreous hemorrhage. The inclusion criteria for these studies were: 1) vitreous hemorrhage in the study eye that had been present for at least one month and that was dense enough to obscure visualization of the fundus on indirect ophthalmoloscopy such that no retinal detail was visible posterior to the equator; 2) best corrected visual acuity (BCVA) worse than 20/200 in the study eye. Patients were randomized in a 1:1:1 ratio to intravitreal injection with saline, 55 IU hyaluronidase and 75 IU hyaluronidase in a 50-ml volume. Clinical ocular assessments were performed at day one, week one and months one, two and three following injection for efficacy and safety outcomes, and at month six and every six months thereafter up to 32 months for safety outcomes.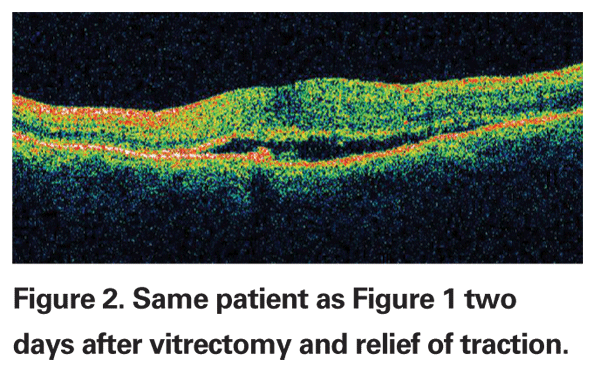
The primary endpoint was clearance of vitreous hemorrhage sufficient to see the underlying pathology and to complete treatment, when indicated, by the month three visit. A patient was considered a treatment success if the vitreous hemorrhage cleared sufficiently for diagnosis of the underlying pathology and one of the following criteria were met within the month three visit: 1) laser treatment of the underlying condition was completed; 2) visualization of the retina revealed that surgery was required and was performed to correct the underlying pathology; or 3) visualization of the macula and a minimum of 180 degrees of the vitreous base and the underlying cause of the hemorrhage resolved without need for further therapy. The secondary endpoints were: 1) a three-line or greater improvement in BCVA; 2) subjective reduction in vitreous hemorrhage density as determined by the study investigator.
A total of 1,125 patients were randomized to 55 IU (n=365), 75 IU (n=377) and saline (n=383) in the two studies. More than 90 percent of patients completed the month three visit. For the primary endpoint, there was a statistically significant improvement at one, two and three months compared to saline for the 55-IU dose. Interestingly, a dose-response was not demonstrated and the 75-IU dose was not statistically significant compared to saline beyond month one. At month three, 32.9 percent of patients achieved the primary endpoint compared to 25.6 percent in the saline group (p=.025). For the secondary endpoint of a three-line or greater improvement in BCVA, there was a statistically significant improvement at months one, two and three for both 55 IU and 75 IU compared to saline. At month three, 44.9 percent of patients in the 55-IU group had a three line or greater improvement in BCVA compared to 34.5 percent in the saline group (p=.004).
Overall, intravitreal injection of hyaluronidase was well-tolerated. Iritis was the most common adverse event, occurring in 62 percent of eyes treated with 55 IU and in 59 percent of eyes treated with 75 IU. A dose response was noted in eyes with moderate or severe iritis; 34 percent with 55 IU and 40 percent with 75 IU. The iritis was often self-limited and did not result in a serious adverse event in any hyaluronidase treated eye.16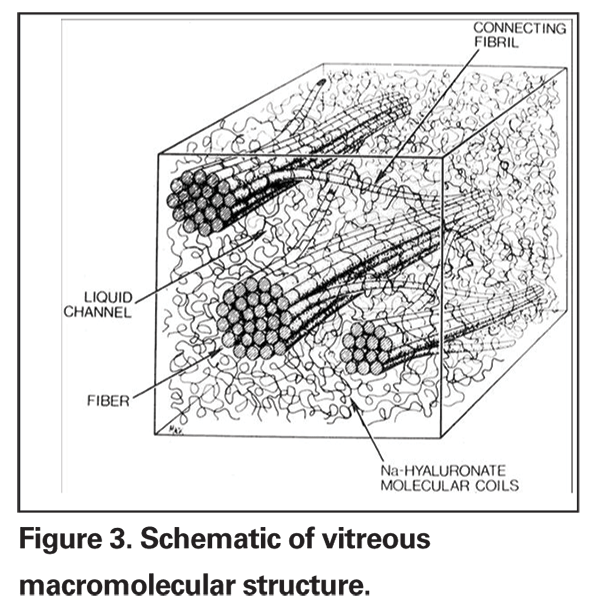
Although the study investigators concluded from these data that intravitreal hyaluronidase was useful in the management of vitreous hemorrhage, hyaluronidase has not received Food and Drug Administration approval for this indication. Hyaluronidase is FDA-approved as a spreading agent to increase the absorption and dispersion of other injected drugs.18 Therefore, at the present time the use of hyaluronidase for vitreous hemorrhage is an off-label indication. Although the treatment effect was statistically significant, the clinical benefit appears modest. For the 55-IU dose recommended by the study investigators, the number needed to treat (NNT) to achieve the primary endpoint is 14, meaning 14 patients must be treated for one to benefit. For the secondary endpoint of a three-line or greater increase in BCVA, the NNT is 10. This may explain the limited acceptance of hyaluronidase as a treatment for vitreous hemorrhage.
Plasmin and Microplasmin
Plasmin is a nonspecific protease which is active in many biologic processes including fibrinolysis, neovascularization and activation of enzymes such as MMP. Plasmin breaks down fibronectin and laminin, thereby creating posterior vitreous detachment in animal models of enzymatic vitreolysis (See Figure 5).17,19-21 Ultra-structural and electrophysiologic studies show no evidence of retinal toxicity with intravitreal injection of plasmin.10,17,19,20,22 Plasmin also creates vitreous liquefaction.10 The combination of posterior vitreous detachment with vitreous liquefaction without retinal toxicity suggests plasmin may be a useful agent for enzymatic vitreolysis. As a result, intravitreal plasmin has been studied in multiple case series involving patients with idiopathic and traumatic macular holes, proliferative diabetic retinopathy, retinopathy of prematurity, pediatric and traumatic retinal detachment and vitreomacular traction syndromes.9,23-29 In most of these case series, autologous plasmin was derived preoperatively from the patient's blood.25,26 Although other techniques for the isolation of autologous plasmin have been described, the most commonly used technique involves drawing 30 ml of whole blood and isolating the plasma. The plasma is then run through a lysine affinity column, which binds only plasminogen. The plasminogen is then eluted off the column, concentrated and then converted by a plasminogen activator into plasmin. The plasmin is then checked for sterility and refrigerated prior to use. Typically, plasmin is injected into the vitreous cavity 20 to 30 minutes prior to vitrectomy.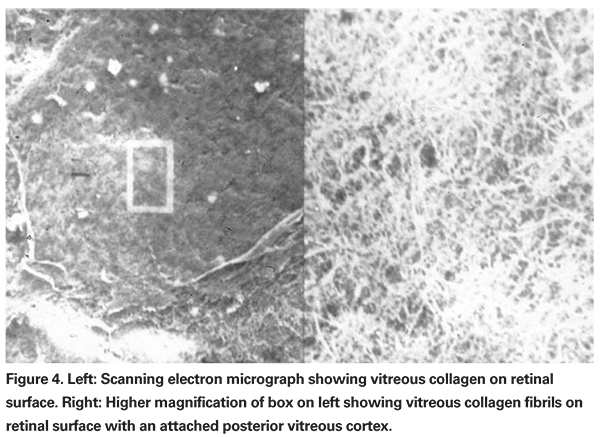
Multiple reports have confirmed that autologous plasmin facilitates intraoperative posterior vitreous detachment and creates vitreous liquefaction. However, the process for isolating autologous plasmin is cumbersome and the enzymatic activity is dependent on multiple variables including the level of plasminogen in the blood. As a result, the amount of enzymatic activity obtained is variable and difficult to quantify.
The development of recombinant human microplasmin alleviates most of the problems associated with autologous plasmin.5,30,31 Microplasmin is a truncated form of plasmin that lacks the 5 kringle domains of plasmin but contains the protease domain making it a smaller molecule than plasmin (27 kD compared to 80 kD) but with similar enzymatic activity. As a recombinant DNA product, microplasmin can be produced in large quantities, allowing for dose-ranging studies on efficacy and safety.
Animal and ex vitro studies demonstrate that microplasmin creates both posterior vitreous detachment and vitreous liquefaction without evidence of retinal toxicity. In an elegant ex vivo study, Dr. Sebag and colleagues demonstrated that microplasmin increased vitreous diffusion coefficients (a measure of liquefaction) in a dose-dependent manner as measured by dynamic light scattering.4 The mechanism of this effect is unknown but may involve breakdown of the macromolecular structure of the vitreous, thereby decreasing bulk viscosity.
Recent work has demonstrated that the mechanical effects of posterior vitreous detachment and vitreous liquefaction induced by enzymatic vitreolysis may result in metabolic changes in the retina. Polly A. Quiram, MD, PhD, and colleagues studied the effect of microplasmin-induced posterior vitreous detachment on vitreous oxygen levels. They found that microplasmin-induced posterior vitreous detachment increased vitreous oxygen levels. They suggested that such an effect may be beneficial in patients with ischemic retinal disease.5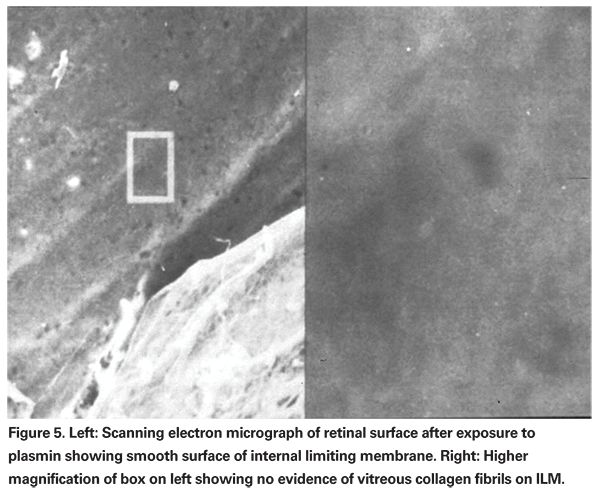
At the present time, microplasmin (ThromboGenics,
An ever-increasing understanding of the importance of the vitreous in the pathogenesis of retinal disease suggests that the safe formation of both posterior vitreous detachment and vitreous liquefaction may have great therapeutic potential in a wide variety of retinal diseases. Recently, Ilse Krebs, MD, and colleagues reported that persistent attachment of the posterior vitreous cortex may be a risk factor for the development of neovascular age-related macular degeneration.3 They speculate that persistent vitreomacular traction may induce chronic low-grade inflammation, maintain macular exposure to cytokines or free radicals in the vitreous gel, or interfere in vitreous oxygenation and nutrition of the macula. They suggest future investigations on the use of pharmacologic vitreolysis to induce posterior vitreous detachment as prophylaxis against neovascular AMD. The future of enzymatic vitreolysis is promising.
Dr. Williams is chair of the Department of Ophthalmology and director of the Beaumont Eye Institute at
Dr. Williams holds intellectual property on the use of plasmin in ocular disease, which has been licensed to NuVue Technologies and ThromboGenics.
1. Sebag J. Anomalous posterior vitreous detachment: A unifying concept in vitreoretinal disease. Graefes Arch Clin Exp Ophthalmol 2004;242:690-698.
2. Williams G. Pharmacologic manipulation of the vitreous during pars plana vitrectomy. In: Alfaro D, Liggett P, eds. Vitreoretinal Surgery of the Injured Eye.
3. Krebs I, Brannath W, Glittenberg C, et al. Posterior vitreomacular adhesion:A potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol 2007;144:741-746.
4. Sebag J. Is pharmacologic vitreolysis brewing? Retina 2002;22:1-3.
5. Quiram P, Leverenz V, Baker R, et al. Microplasmin-induced posterior vitreous detachment affects vitreous oxygen levels. Retina 2007;27:1090-1096.
6. Gandorfer A. Pharmacologic Vitreolysis. Dev Ophthalmol 2007;39:1490156.
7. Hageman G, Russell S. Chondroitinase-mediated disinsertion of the primate vitreous body. Invest Ophthalmol Vis Sci 1994;35:1260-1268.
8. Hesse L, Kroll P. Enzymatically induced posterior vitreous detachment in proliferative diabetic retinopathy. Klin Monatsbl Augenheilkd 1999;214:84-89.
9. Trese M, Williams G, Hartzer M. A new approach to stage 3 macular holes. Ophthalmology 2000;107:1607-1611.
10. Staubach F, Nober V, Janknecht P. Enzyme-assisted vitrectomy in enucleated pig eyes: a comparison of hyaluronidase, chondroitinase, and plasmin. Curr Eye Res 2004;29:261-268.
11. Tezel T, Del Priore L, Kaplan H. Posterior vitreous detachment with dispase. Retina 1998;18:7-15.
12. Sebag J. Anatomy and pathology of the vitreoretinal interface. Eye 1992;6:541-552.
13. Sebag J. Molecular biology of pharmacologic vitreolysis. Trans Am Ophthalmol 2005;103:473-494.
14. Sethi C, Bailey T, Luthert P, Chong N. Matrix metalloproteinase biology applied to vitreoretinal disorders. Br J Ophthalmol 2000;84:654-666.
15. Kuppermann B, Thomas E, de Smet M, et al. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol 2005;140:573-584.
16. Kuppermann B, Thomas E, De Smet M, et al. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol 2005;140:585-597.
17. Wang Z, Zhang X, Xu X, et al. PVD following plasmin but not hyaluronidase: Implications for combination pharmacologic vitreolysis therapy. Retina 2005;25:38-43.
18. fda.gov. FDA approves Vitrase (Hyaluronidase for injection) May 2004.
19. Verstraeten T, Chapman C, Hartzer M, et al. Pharmacologic induction of posterior vitreous detachment in the rabbit. Arch Ophthalmol 1993;111:849-854.
20. Hermel M, Schrage N. Efficacy of plasmin enzymes and chondroitinase ABC in masked, placebo-controlled in vivo study. Graefes Arch Clin Exp Ophthalmol 2007;103:473-494.
21. Hikichi T, Yanagiya N, Kado N. Posterior vitreous detachment induced by injection of plasmin and sulfur hexafluoride in the rabbit vitreous. Retina 1999;19:55-58.
22. Gandorfer A, Putz E, Welge-Lussen U. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br J Ophthalmol 2001;85:6-10.
23. Azzolini C, D'Angelo A, Maestranzi G. Intrasurgical plasmin enzyme in diabetic macular edema. Am J Ophthalmol 2004;138:560-566.
24. Gandorfer A, Ulbig M, Kampik A. Plasmin-assisted vitrectomy eliminates cortical vitreous remnants. Eye 2002;16:95-97.
25. Trese M. Enzymatic-assisted vitrectomy. Eye 2002;16:365-368.
26. Rizzo S, Pellegrini G, Benocci F, et al. Autologous plasmin for pharmacologic vitreolysis prepared 1 hour before surgery. Retina 2006;26:791-796.
27. Williams J, Trese M, Williams G, Hartzer M. Autologous plasmin enzyme in the surgical management of diabetic retinopathy. Ophthalmology 2001;108:1902-1905.
28. Wu W, Dresner K, Trese M, et al. Pediatric traumatic macular hole: Results of autologous plasmin enzyme-assisted vitrectomy. Am J Ophthalmol 2007;144:668-672.
29. Margherio A, Margherio R, Hartzer M, et al. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology 1998;105:1617-1620.
30. Sebag J, Ansari R, Suh K. Pharmacologic vitreolysis with microplasmin increases vitreous diffusion coefficients. Graefe's Arch Clin Exp Ophthalmol 2007;245:576-580.
31. Chen W, Huang X, Ma X, et al. Enzymatic vitreolysis with recombinant microplasminogen and tissue plasminogen activator. Eye 2007;2007:1-8.