When faced with treating a new glaucoma patient, there are two important predictors of patient outcomes to consider. First, one must determine the stage of disease—i.e., how advanced the patient’s disease is at the time of presentation. Second, one needs to determine the patient’s risk of progression. These are two of the primary determinants of the type and extent of treatment we offer.
Staging the Disease
There are two reasons it’s important to accurately stage the disease. First, as already noted, accurately staging the disease informs the type of clinical care we provide, as described in the American Academy of Ophthalmology’s Preferred Practice Patterns. In particular, advanced disease in a presenting patient should impact our choice of treatment because of what we learned from the CIGTS (Combined Initial Glaucoma Treatment Study) trial, led by Paul Lichter, MD. That study showed that late-stage glaucoma patients do better over the long term (in terms of visual field preservation) if they have a trabeculectomy as their initial treatment. Thus, when we encounter a patient with advanced disease, we’re justified in considering an aggressive initial treatment such as trabeculectomy.
The second reason it’s important to accurately stage the disease is that there are billing and coding ramifications. If your patient’s glaucoma is mild or moderate, a MIGS procedure is reimbursable; in contrast, if the glaucoma is severe, a MIGS procedure won’t be reimbursed. Similarly, OCT nerve fiber layer testing isn’t reimbursable if your patient has severe stage-three glaucoma. So staging the disease correctly is important if you hope to get reimbursed for any procedures you perform.
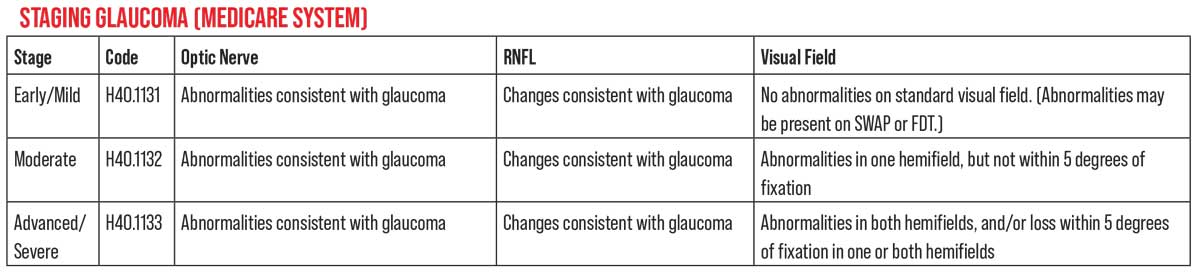
Medicare defines three stages of glaucoma. However, it’s important to remember that this staging isn’t based on findings from any specific clinical trial. (While billing and coding should comply with Medicare definitions in order to obtain proper reimbursement, our clinical decisions should be informed by data and definitions from the landmark clinical trials, in order to achieve the best outcomes.)
For coding purposes, H40.1131 is used for early or mild-stage glaucoma. This is defined as optic nerve abnormalities consistent with glaucoma, and retinal nerve fiber layer changes, but with no visual field abnormalities. (The exception would be abnormalities only present on SWAP or FTD visual field testing.)
H40.1132 is used for moderate-stage glaucoma. Medicare defines that as optic nerve abnormalities consistent with glaucoma and retinal nerve fiber layer changes, plus visual field abnormalities in one hemifield—but not within 5 degrees of fixation.
H40.1133 is used for advanced/severe-stage glaucoma, defined as optic nerve abnormalities consistent with glaucoma, retinal nerve fiber layer changes, glaucomatous visual field abnormalities in both hemifields and/or vision loss within 5 degrees of fixation in at least one hemifield. The difference between these definitions and clinical-trial-based definitions can be seen by considering how advanced disease is defined in the CIGTS study: having a mean deviation greater than -10 dB on a visual field.
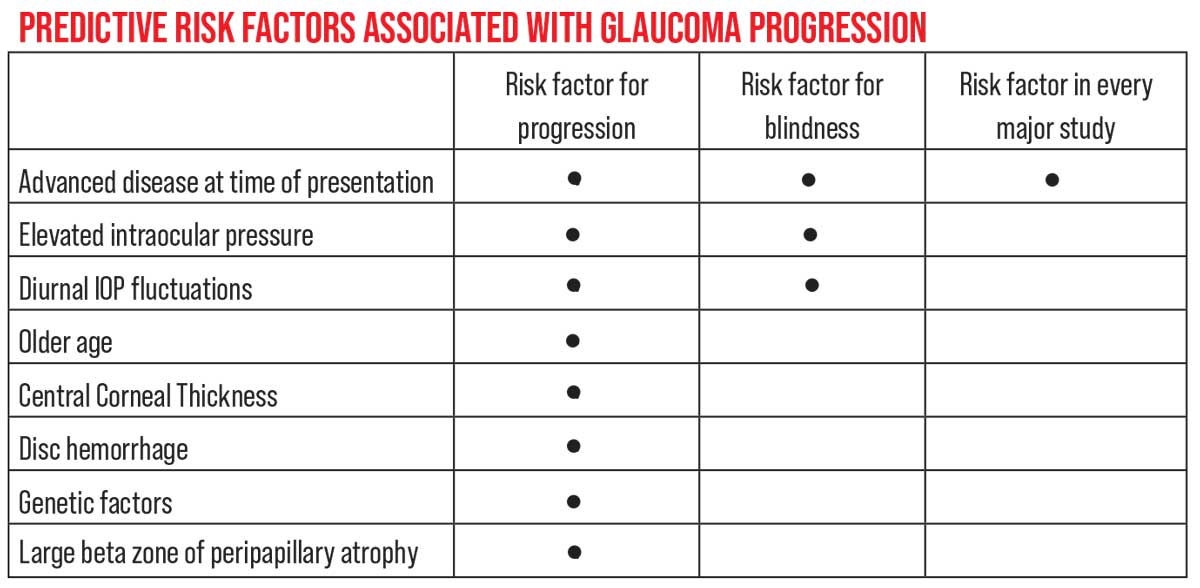 |
| Although numerous risk factors have been found to be associated with glaucoma progression in different studies, only one has been associated with progression in every major clinical trial: advanced disease at the time of presentation. |
Glaucoma Pathophysiology
To understand the staging of glaucoma, it helps to review the pathophysiology of the disease. Glaucoma can be defined as a pathologic condition in which there’s a progressive loss of ganglion cell axons leading to visual field deficits, with elevated intraocular pressure as a known major risk factor.
We know that the primary damage occurs at the lamina cribrosa. What we don’t know for certain is exactly how this damage occurs. Both structural and vascular damage are seen as the disease progresses, and it’s not yet clear which one comes first. That’s led to two possible explanations for the development of the damage: the mechanical theory and the vascular theory.
Because the entry point of the ganglion cell axons at the lamina cribrosa is the structural weak point of the eye, the mechanical theory proposes that barotrauma resulting from the increased intraocular pressure found in glaucoma squashes the lamina, with both compressive and shearing forces causing damage. Because the ganglion cell axons pass through the laminar plates, they get stretched as the laminar plates are pulled apart.
To put it another way, the physiologic consequence of elevated IOP is inhibition of axoplasmic flow at the lamina cribrosa; the functional consequence of axonal loss (or dysfunction) is decreased sensitivity to light. It’s postulated that this damage then leads to diminished vascularization of this area; when tissue is dying, the body will be conservative and not send new blood vessels there. This theory has been suggested by Harry Quigley, MD, Jost B. Jonas, MD, and Douglas Anderson, MD.1,2
The vascular theory, favored by Jack Cioffi, MD,3 suggests that the vascular damage comes first, triggering the structural damage. This theory suggests that vascular dysfunction is caused by either the capillaries in the lamina cribrosa area losing some of their ability to autoregulate, or a problem with the glial cells that support the vessels, the periocytes. Periocyte loss then leads to an infarction. If this theory is correct, then this infarction leads to loss of tissue and the collapse of the lamina.
At this point, we don’t know whether the mechanical or vascular theory is correct. It’s most likely that the damage caused by glaucoma is multifactorial; in fact, both theories may be correct.
One way to look at glaucomatous disease is as an acceleration of the natural aging process, a process that leads to a loss of vision over time. A healthy eye experiences a loss of about 0.5 percent of ganglion cell axons per year as we age, as shown in research done by Rosario Hernandez-Neufeld, DDS. That’s one of the reasons that, even if there’s no disease present, you find decreased contrast sensitivity, reduced levels of spatial recognition and challenges with light-to-dark and dark-to-light adaptation as we age. This is one reason that older people don’t see as well as they did when younger, even if they’re considered healthy. Glaucoma, we believe, causes a hyper-acceleration of that aging process in patients with the disease.
 |
Determining Extent of Damage
To stage the disease, in addition
to a thorough physical exam of the patient’s eyes, we have to evaluate the patient’s condition using the best tools at our disposal. For most clinicians today, that means obtaining objective structural data by scanning the retina and optic nerve using optical coherence tomography, as well as performing visual fields to get a more subjective measure of visual performance. As most ophthalmologists know, structural and functional testing—the former objective and the latter subjective—often don’t agree regarding the patient’s condition.
The earliest that standard visual field testing can pick up glaucomatous damage is when there’s a 30- to 45-percent loss of ganglion cells. This was verified in two key studies. The first was a study conducted by Harry Quigley, MD, that looked at the histology of several eyes that had visual fields done shortly before the patients died.4 The second was done by Robert Harwerth, OD, PhD, using a colony of monkeys.5 He trained them to do visual field testing and then induced an experimental form of glaucoma, and was able to verify what Dr. Quigley had found. This inability to demonstrate loss until 35 to 40 percent of ganglion cells are gone is a key reason that structural testing and visual fields don’t always correlate.
The European Glaucoma Prevention Study and OHTS study made it clear that we often pick up structural optic nerve or nerve fiber layer damage earlier than visual field loss. In the OHTS study, 50 percent of patients’ progression was detected by structural optic nerve criteria;6 in the EGPS study, it was 40 percent.7 However, it must be noted that even though 50 percent progressed by optic nerve criteria before the visual field revealed the loss in the OHTS study, the visual field picked up the problem first in the other 50 percent of patients.
From a practical perspective, this means that if you note a visual field change that’s highly suggestive of glaucoma—specifically, a visual field defect that localizes to the retinal nerve fiber layer—then treatment is indicated. Glaucoma doesn’t always present with anatomical changes on structural imaging; the visual field can sometimes be the first indication of pathology. (This ties into the evolving concept called “green disease,” where even though a patient’s “normal/abnormal” OCT chart shows all green—suggesting that the readings are all within the normal range—the patient actually does have glaucomatous disease. You may find the evidence on visual field testing, or in other evidence picked up by OCT, such as nerve fiber layer thinning and/or asymmetry over time.)
Risk Factors for Progression
Risk factors for progression are another important determinant when evaluating a patient for glaucoma. They allow us to approximate the likelihood that the patient’s vision will deteriorate in the near future (and long-term). Obviously, our treatment plan will be different when a patient has multiple risk factors for progression, as opposed to the patient having only one or two risk factors.
Different clinical studies have helped us identify numerous risk factors for glaucoma progression, including elevated IOP, significant diurnal IOP fluctuations, older age, reduced central corneal thickness, the presence of disc hemorrhages, certain genetic factors (for example, mutations in the myocilin, WDR-36 or OPTN genes, or single-nucleotide polymorphisms in the myocilin gene promoter), and a large beta zone of peripapillary atrophy. However, only one risk factor has been found to be statistically significant in every study: advanced disease at the time of presentation.8-12 (It’s also one of three risk factors associated with glaucoma-related blindness, along with elevated IOP and significant diurnal IOP fluctuations.) Even family history hasn’t been a significant predictor of progression in every study; it wasn’t a significant risk factor in the Ocular Hypertension Treatment Study, for example.
It’s not obvious why the other risk factors don’t have a significant association with progression in every study. However, it’s easy to guess why advanced disease consistently turns up as a powerful risk factor; once the disease is advanced, the tissue is already badly compromised. This damaged tissue is highly vulnerable to additional barotrauma. Ironically, if our treatments for glaucoma were more reliable and effective—if we were able to halt the disease no matter what stage it’s at—then advanced stage of disease wouldn’t be such a powerful risk factor.
In terms of stratifying risk, data from the Ocular Hypertension Treatment Study, led by Michael Kass, MD, at Washington University School of Medicine in St. Louis, has led to the so-called “rule of five.” The study found that you’re at high risk of developing glaucomatous disease within a five-to-10-year period if you have any or all of these factors: a cup-to-disc ratio greater than 0.5; central corneal thickness thinner than 555 µm; and/or an IOP higher than 25 mmHg—even if there’s no evidence of disease. There’s also an online risk calculator developed by the ocular hypertension treatment group (ohts.wustl.edu/risk/) that was verified by Felipe Medeiros, MD.13
While the practicality of providing patients with a specific risk-of-glaucoma number using the calculator is debatable, it remains true that the “rule of five” risk factors play a crucial role in clinical decision-making when managing patients with ocular hypertension.
Imperfect, But Effective
To provide our patients with the most appropriate and effective care, we have to be able to evaluate their current and projected future status, as it relates to their glaucomatous disease, and we have to do so as accurately as possible. Our current tools and understanding are imperfect, but by applying them effectively to our clinical decision-making, we’ve made great strides in preserving vision for our patients.
Dr. Rhee is chair of the Department of Ophthalmology and Visual Sciences at University Hospitals Cleveland Medical Center; director of the Eye Institute at University Hospitals; division chief of ophthalmology at University Hospitals Ahuja Medical Center; and a professor at the Case Western Reserve University School of Medicine. He receives research funding from Allergan, Glaukos and Ivantis; he is on the scientific advisory board for Aerie and Alcon, the speaker’s bureau for Aerie, Bausch + Lomb and Ivantis, and the data safety monitoring board for Ocular Therapeutix.
Dr. Sozeri is an assistant professor of ophthalmology at Case Western Reserve University and University Hospitals. She’s a member of the glaucoma service and the associate glaucoma fellowship director.
Dr. Lau is the 2021-2022 Case Western Reserve University and University Hospitals glaucoma fellow. Drs. Lau and Sozeri have no financial disclosures to report.
1. Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 1981;99:4:635-49.
2. Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 2003;44:12:5189-95.
3. Zhao DY, Cioffi GA. Anterior optic nerve microvascular changes in human glaucomatous optic neuropathy. Eye (Lond) 2000;14 (Pt 3B):445-9.
4. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 1989;107:5:453-64.
5. Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci 1999;40:10:2242-50.
6. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:6:714-20; discussion 829-30.
7. Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I; European Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma Prevention Study. Ophthalmology 2005;112:3:366-75.
8. Forsman E, Kivelä T, Vesti E. Lifetime visual disability in open-angle glaucoma and ocular hypertension. Journal of Glaucoma 2007;16:3:313-9.
9. Chen PP. Risk and risk factors for blindness from glaucoma. Curr Opin Ophthal 2004;15:2:107-11.
10. Oliver JE, Hattenhauer MG, Herman D, et al. Blindness and glaucoma: A comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am J Ophthalmol 2002;133:6:764-72.
11. Grant WM, Burke JF. Why do some people go blind from glaucoma? J Ophthalmology 1982;89:9:991-8.
12. Rosenberg LF. Glaucoma: Early detection and therapy for prevention of vision loss. Am Fam Physician. 1995;52:8:2289-98, 2303-4.
13. Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol 2005;123:10:1351-60.



