When cells are stressed by a disease such as glaucoma, their mitochondria produce elevated levels of flavoprotein fluorescence. FPF mapping, a new, non-invasive ocular imaging technology, measures the FPF levels in the eye, providing a way to evaluate the level of metabolic oxidative stress the cells are undergoing. This is showing promise as a way to diagnose glaucoma earlier than previously possible, determine whether a therapy has been effective, distinguish between healthy ocular hypertensives and early glaucoma patients, and objectively measure the damage already present in glaucomatous eyes.
A recently published retrospective, cross-sectional study conducted at the New York Eye and Ear Infirmary of Mount Sinai is the latest to demonstrate the potential utility of this technology.1 This study measured FPF levels in 86 eyes—50 eyes with POAG and 36 healthy eyes. The data showed that FPF was significantly higher in POAG eyes than in healthy eyes (mean SD: 46.4 ±27.9 versus 28.0 ±11.7, p<0.001).
 |
|
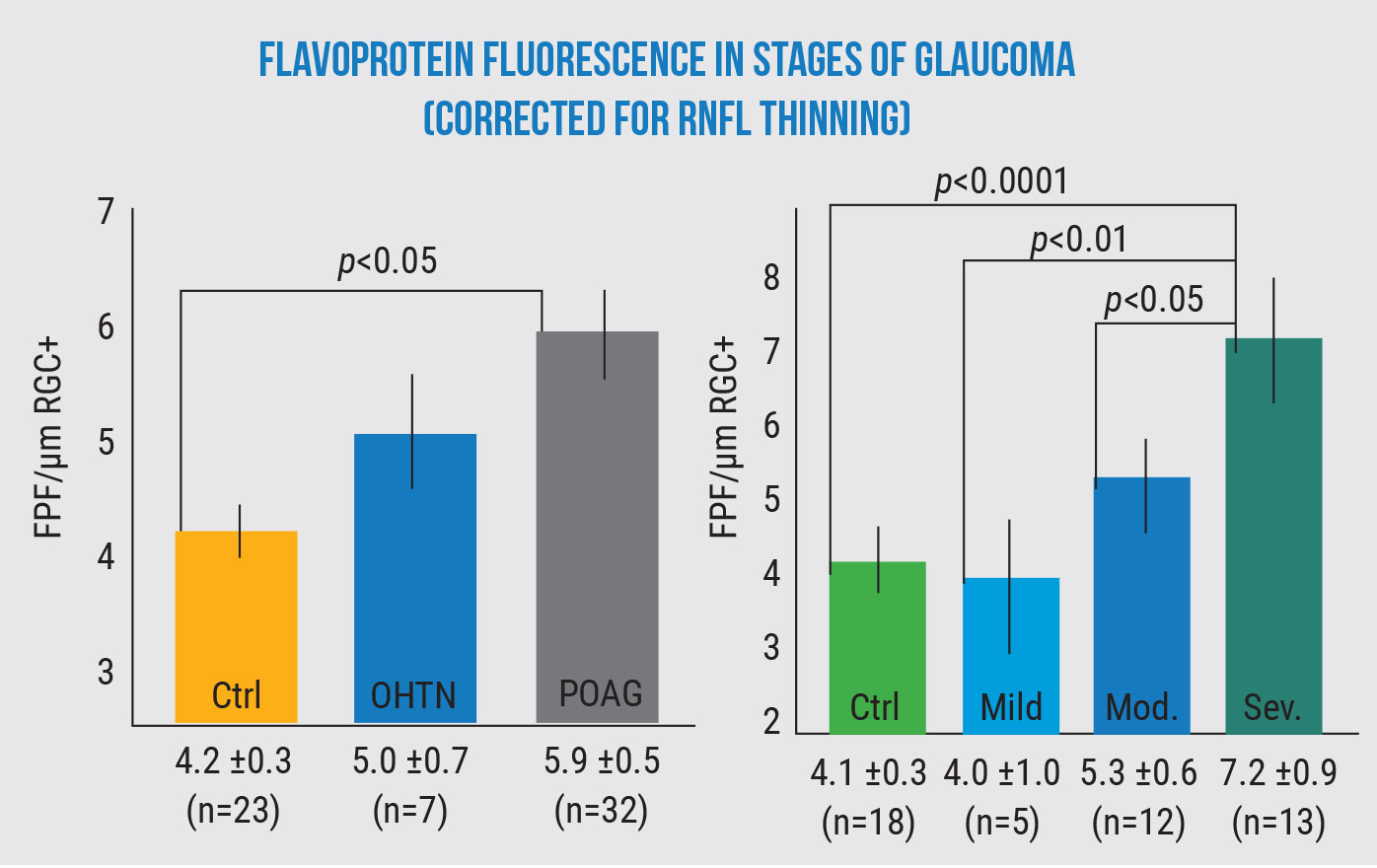
Flavoprotein fluorescence increases as glaucoma gets worse and increasingly stresses retinal cells. (All comparisons ANCOVA p<0.01.) Geyman et al, 2018.2 |
In addition, the researchers noted that in eyes with POAG, the level of FPF correlated with visual field mean deviation (p<0.001), visual field pattern standard deviation (p=0.003) and cpRNFLT (p=0.001). This suggests that FPF mapping might offer a substitute or adjunct to visual field testing. (Visual field testing, which is the current gold standard for measuring visual function, is tedious and unpopular with patients. Its subjective nature contributes to a variety of potential artifacts.)
Richard B. Rosen, MD, vice chair and director of ophthalmology research at the New York Eye and Ear Infirmary and chief of the retina service for the Mount Sinai Health System, was principal investigator for the study. “Levels of flavoprotein fluorescence are measured with a very sensitive detector built into the OcuMet Beacon automated fundus camera,” he explains. “This is a commercial device developed by a company called OcuSciences, which is a spinoff from the University of Michigan. The camera has very narrow notch filters, so it detects a very specific wavelength of fluorescence, shorter than the typical lipofuscin autofluoresence most ophthalmologists are familiar with.
“What’s interesting is, unlike lipofuscin autofluorescence, which tells us that cells have died because they’ve stopped fluorescing, the level of FPF changes with the condition of the cells. It increases when the cells are stressed and decreases as they become healthier. The measurement is objective, and studies have indicated that it has good repeatability: Measuring the same patient multiple times produces results within a few percentage points each time.
“We’ve previously published data about a group of diabetic patients receiving anti-VEGF treatment,” he continues. “The visual acuity changes in the treated patients correlated with the measured levels of FPF—better than they correlated with the structural changes seen on OCT. Of course, that’s always been a problem with OCT: There isn’t a great correlation between structural thickness and visual function. That’s why the FDA doesn’t consider nerve fiber layer thickness to be a good primary endpoint in studies, although it may be used as a surrogate endpoint. In contrast, the FPF measure seems to correlate well with function and functional changes in glaucoma patients. Based on that, it has some promise.”
Dr. Rosen says this technology has the potential to be clinically useful in a number of ways. “First, it may help us diagnose glaucoma patients earlier than previously possible,” he says. “Frequently, we diagnose glaucoma after damage has occurred. My idea of where this new measure might fit in is if we see early changes suggestive of glaucoma, and the levels of FPF are high, that might make us lean in the direction of making an earlier diagnosis of glaucoma. It’s sort of pre-structural-damage metabolic information. This wouldn’t be diagnostic by itself, but it could be very useful taken in context.
“Similarly, this technology might allow us to distinguish between ocular hypertensives and early glaucoma patients,” he says. “As every ophthalmologist knows, some patients have high pressures but don’t really have glaucoma. These ocular hypertensives probably don’t need to be treated. A measure that quantifies the metabolic stress that’s occurring could make it possible to tell whether or not the high pressure is causing a problem. Studies suggest this technology could provide a means to do that.2
“Second, it may help us determine the level of existing damage,” he says. “Our latest study looked at a group of glaucoma patients with different levels of the disease and compared their data to normals. It showed that there was good correlation between some recognized standards of visual field changes and FPF levels, making it a potential tool for grading the level of glaucoma.
“Another potential use, as noted earlier, is that this technology might help us determine whether our therapy was effective,” he says. “Did the patient respond to our treatment? For example, studies have demonstrated that in a diabetic patient with a high level of FPF, the FPF level goes down after you inject them with anti-VEGF or steroids.3 In another example, Robert Ritch, MD, did a small study he reported at the annual meeting of the American Glaucoma Society a few years ago, in which he showed that you could decrease the FPF by giving the patient a combination of antioxidant nutraceuticals.
“Finally, this new measure might enable us to tell whether the pressure we’ve targeted has actually quieted the disease process,” he continues. “We know that even if we completely control the pressure, many people will continue to lose vision; this measurement might help us determine how low we need to go. These kinds of uses probably represent the future for this technology.”
 |
|
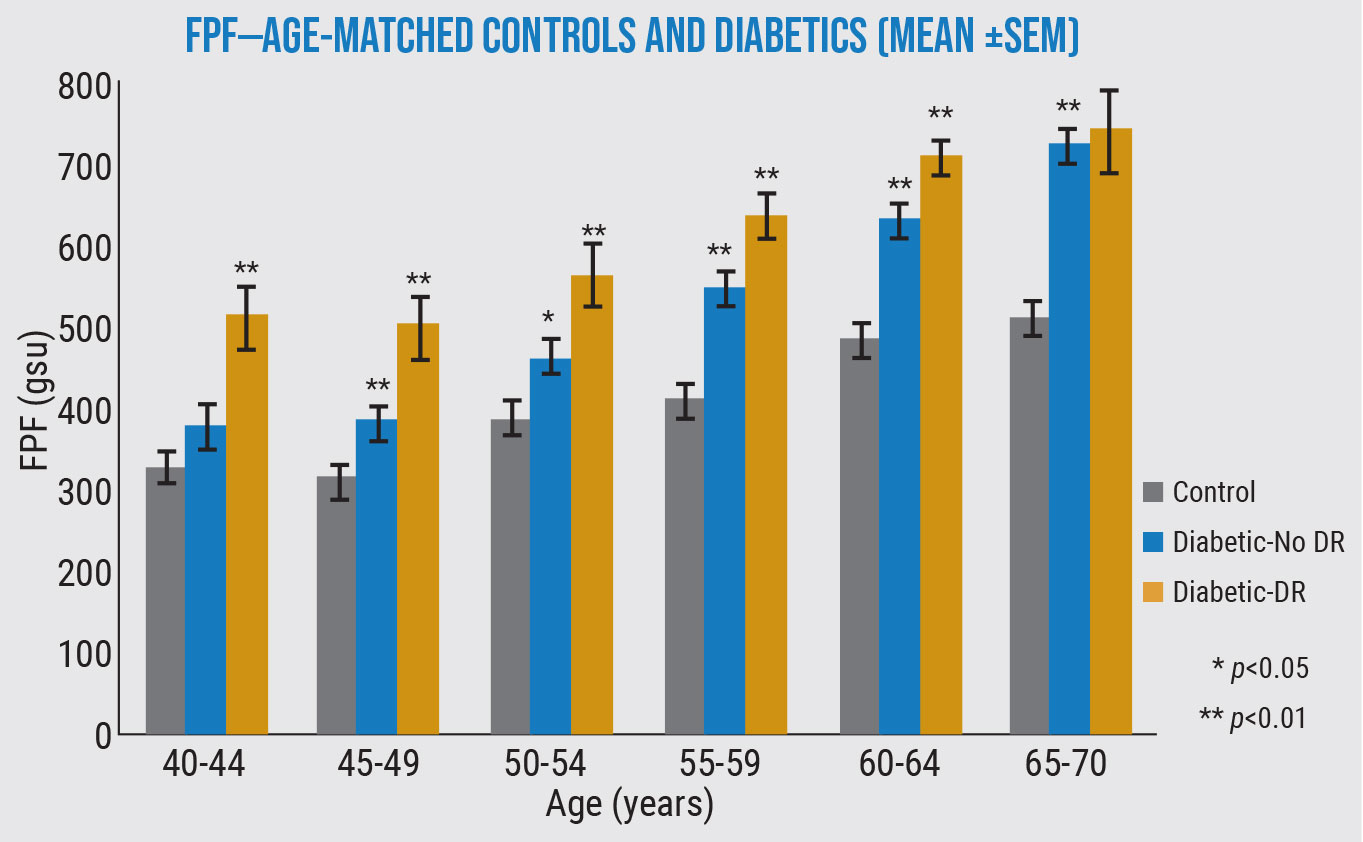
In this study, the levels of flavoprotein fluorescence were significantly higher in most diabetic eyes than healthy eyes—and even higher in diabetic eyes with diabetic retinopathy—in every age group. (Unpublished data from Matthew G. Field, MD, Victor M. Elner, MD, PhD, and Donald G. Puro, MD, PhD.) |
Dr. Rosen explains that the FPF measure works even in cases of advanced glaucoma, although one confounding factor needs to be taken into account. “In an earlier study2 we found that the thickness of the nerve fiber layer has an impact on the amount of fluorescence,” he explains. “This makes sense physiologically, because if you have less tissue to fluoresce, you’ll get less fluorescence. Some of the advanced patients didn’t appear to have the higher fluorescence we expected to find until we factored in the thinner nerve fiber layer. When we compensated for that, we found a very good linear correlation between the disease and the FPF level. So it appears that in more advanced patients, we’ll need an algorithm that corrects for a thinner nerve fiber layer.”
Dr. Rosen says metabolic imaging is an area of great interest right now. “Groups in Iceland and Germany have been championing the idea of measuring metabolic activity with oximetry,” he says. “I think measuring metabolic activity will be the future. It will allow us to detect disease without having to wait for a loss of tissue or function. This is the holy grail for retinal imaging.
“At this point, many questions remain to be answered,” he admits. “The technology has become much smaller and more affordable since I started working with the prototype 10 years ago, but we still have to maximize the human interface. And we don’t know exactly how sensitive the measure is. We’ve seen patients come in with very high IOPs and high levels of FPF. We bring the IOP to normal and the levels of FPF normalize. But how well does the level of FPF correlate to smaller changes of 3 or 4 mmHg? What’s the smallest change in IOP that this is sensitive to? Those are questions we don’t have the answers to yet.”
Dr. Rosen says several investigators have already expressed interest in participating in further testing. “This device is currently in a number of centers, including Stanford University and the Cole Eye Institute,” he says. “We’re having conversations with different glaucoma groups around the country about doing studies. Of course, seeing how this plays out in the clinical sphere will take a lot of work, in terms of enrolling patients who are well-characterized with different levels of glaucoma. Right now, we just have proof of concept. A lot of things get to this stage and look very promising, but in the end, it’s hard to tell whether they’ll actually make it to the ophthalmologist’s lane.”
Dr. Rosen notes that even if this measure turns out to be more sensitive than visual fields, it could take a while to fall into common usage. “People are comfortable with what they know, so it would probably start out as a supplement to visual fields and OCT. But if it continues to produce these kinds of results, I believe the future of this technology is bright.”
1. Zhou DB, Castanos MV, Geyman L, et al. Mitochondrial dysfunction in primary open-angle glaucoma characterized by flavoprotein fluorescence at the optic nerve head. Ophth Glaucoma 2022;5:4:413-420.
2. Geyman LS, Suwan Y, Garg R, et al. Noninvasive detection of mitochondrial dysfunction in ocular hypertension and primary open-angle glaucoma. J Glaucoma 2018;27:7:592-599.
3. Andrade Romo JS, Lynch G, Liu K, et al. Flavoprotein fluorescence correlation with visual acuity response in patients receiving anti-VEGF injection for diabetic macular edema. Oxid Med Cell Longev 2018:3567306.




