As you know, careful preoperative planning is essential to succeed with cataract surgery. Whether we’re inquiring about the use of blood thinners, assessing a patient’s ability to lie flat or identifying anatomical changes such as weak zonules or a shallow anterior chamber, our success relies heavily on finding sources of potential complications before surgery and adjusting our approach as necessary.
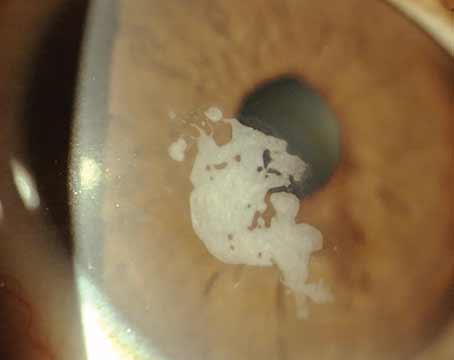
One major difficulty is the presence of corneal pathology that causes opacification and hinders a clear view through the cornea to the lens, increasing the likelihood of inaccurate IOL calculations. Common causes of this type of corneal pathology include a central scar, severe corneal surface disease, limbal stem cell deficiency and endothelial cell dysfunction. In this article, we’ll address each of these sources of “cloudy” corneas and provide tips to help you confidently respond to the challenges they present.
Optimizing the Ocular Surface
Before performing IOL calculations for a patient with a cloudy cornea, you need to treat ocular surface a disease adequately. This step helps ensure accurate biometry and topography readings, which can change significantly before and after treatment. Additionally, treatment of ocular surface disease not only improves visualization during surgery but also decreases the risks that recalcitrant disease might pose after surgery. Most important, control of OSD will lead to a better overall refractive outcome and higher patient satisfaction.
 |
Keratometry is essential to mapping out a good approach to these patients. Looking at the placido rings helps to diagnose subtle cases of epithelial basement membrane disease, for example. If necessary, perform epithelial debridement or excimer laser phototherapeutic keratectomy at least three months before obtaining final IOL calculations for these patients.1 Failure to identify the presence of EBMD will lead to inaccurate IOL selection and dissatisfied patients with poor visual outcomes.
Less common but more serious conditions that can lead to poor visualization include Stevens-Johnson syndrome2 and graft-versus-host disease. Preoperative preventative and stabilizing measures are the keys to achieving satisfactory visual outcomes in these cases. In both SJS and graft-versus-host, inflammation leads to changes, predisposing patients to severe dryness, conjunctival compromise, deficient tear production and eyelid margin disease. Because surgical manipulation can exacerbate the disease process, you must aggressively control any surface disease or existing inflammation.
Treatments to Consider
Consider a broad armamentarium when preparing the ocular surface for surgery. You can try frequent use of preservative-free artificial tears, topical corticosteroids, topical 0.05% cyclosporine (Restasis, Allergan) or 0.5% lifitegrast (Xiidra, Shire) and/or serum tears.
These treatments may not be enough, however, and the use of amniotic membrane grafting, bandage contact lenses, tarsorraphy or the PROSE (prosthetic replacement of the ocular surface ecosystem) Lens (BostonSight) may be useful in severe cases, both before and after surgery.
 |
Patients with a history of ocular herpes simplex or herpes zoster infection should be carefully educated about the increased chance of disease reactivation.3
In general, patients should not have evidence of active infection for at least three months before surgery.4 No clear guidelines exist for antiviral prophylaxis. However, for patients who aren’t already receiving treatment, consider starting them on prophylactic dosing (depending on the type of previous infection) for at least one week before surgery. Continue this therapy for up to six months after surgery, until discontinuing topical steroids.
Besides employing these strategies, you may need to attempt limbal stem cell transplantation to optimize the surface and the view through the cornea before surgery in cases of limbal stem cell deficiency and severe corneal conjunctivalization.
Preoperative Planning
Once control of preexisting pathologies is optimized to the greatest degree possible, visualization through a cloudy cornea should improve and can be maximized with specific surgical strategies.
As with all cataract surgeries, proper lens selection is the key to achieving a good outcome. However, corneal opacities that persist even after the surface has been optimized can prevent accurate biometry readings, although this is true to a lesser extent with newer optical biometry systems. If you’re concerned that biometry findings aren’t accurate, you can use average K-readings (~45) or keratometry from the fellow eye as a reasonable alternative.
You should strongly consider using a three-piece IOL; such lenses provide several options in the event of anterior or posterior capsular compromises, including placement of the IOL in the sulcus, with or without optic capture and scleral fixation.
Surgically Speaking
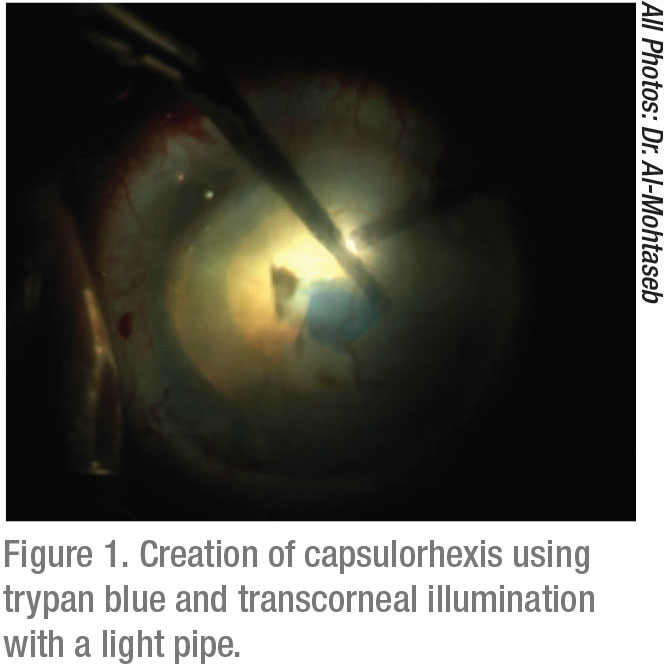
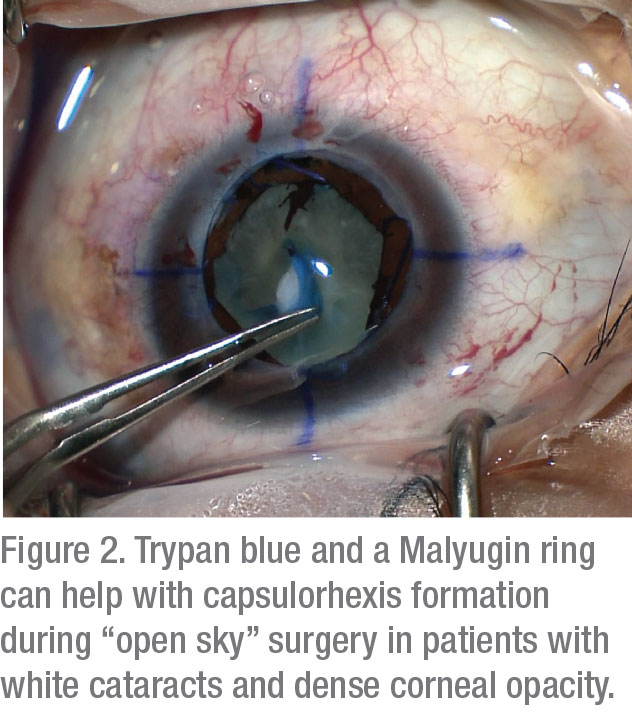
In the operating room, two tactics have been shown to improve visualization: capsular staining and lighting adjustments. One study reported that the use of 0.06% trypan blue greatly improves BCVA in patients with corneal opacities.5 Although instillation of this stain can make the capsule brittle, the payoff of improved visualization greatly outweighs the insignificant risks of this side effect.
Lighting adjustments that can improve visualization include the use of low to medium settings to reduce backscatter. In cases of significant corneal opacification, transcorneal oblique illumination is ideal.6 The use of this newer strategy can greatly improve the view of the anterior chamber. Since it was first described,7 this method has been shown to increase success in a wide range of anterior segment surgeries. The technique requires the use of a small-gauge light source, usually the light pipe used from a vitrectomy machine. The chandelier illumination is placed in the paracentesis; when used during capsular staining, it allows you to comfortably create a capsulorhexis and perform phacoemulsification.
To decrease the risk of anterior capsular tear or radialization during rhexis construction in your patient’s eye, consider steps that can reduce posterior pressure:
• proper patient positioning;
• globe softening techniques;
• hyperosmotic agents; and
• an anterior vitrectomy.8
Additionally, start the rhexis with optimal visualization far from the opacity to ensure ideal initiation and control before proceeding toward areas with a poorer view.
Endothelial Challenges
In cases of endothelial dysfunction, most commonly seen in Fuchs’ dystrophy concomitant with a visually significant cataract, we recommend performing combined procedures involving phacoemulsification and Descemet’s stripping endothelial keratoplasty (DSEK)9 or Descemet’s membrane endothelial keratoplasty (DMEK).10
Combined approaches are more cost-effective, hasten visual recovery and prevent the need for multiple surgeries. One large study found no difference in complication rates, endothelial cell loss, graft success or visual improvement associated with either of these approaches, compared to sequential surgery (DMEK followed by cataract surgery).11 Sequential surgery can also be considered in patients with significant astigmatism, as long as the cornea is stable before the IOL measurements and toric IOL implantation.
However, if you’ve got a patient who definitely needs a corneal transplant, but you’re considering whether or not he’d benefit from a cataract procedure at the same time, keep in mind that endothelial transplant procedures alone can increase the risk of cataract formation, especially in patients over 50 who have shallow anterior chambers.12
Therefore, a transplant procedure alone should be considered more strongly in patients who have a lower risk of cataract formation (younger patients and patients with deep ACs). In older patients or those with shallow ACs, doing a combined procedure could be a better choice because earlier cataract formation is more of a risk in those individuals.
Combination Surgery Tips
If you combine DSEK/DMEK and cataract surgery in these patients, use cohesive viscoelastics to minimize interference with the graft, keeping in mind that dispersives are more difficult to remove completely. Avoid IOL optic damage to the graft by constructing a slightly smaller capsulorhexis and using intracameral miotics, minimizing anterior displacement of the IOL. Finally, note that hyperopic shifts have been noted after DSEK and DMEK.13 A more myopic refractive target (-0.5 D to -1D for DMEK and -0.75 D to -1.5 D for DSEK) can facilitate emmetropia, if desired.
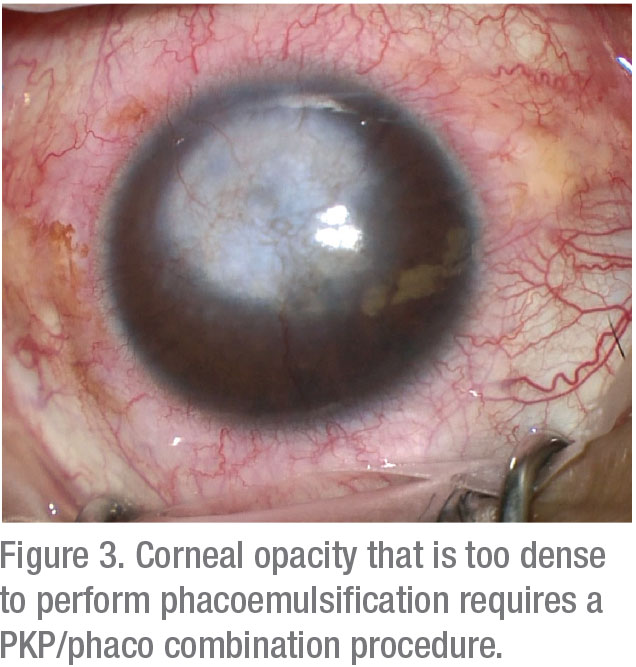 |
For patients with severe corneal scarring and opacification, combining penetrating keratoplasty with cataract extraction offers the expected benefits of preventing the need for a second surgery and decreasing the risk of endothelial cell loss.
However, understand that postoperative refractive outcomes are unpredictable and can be significantly disappointing after using this approach. One study demonstrated only a 62-percent rate of emmetropia within 2 D.14
Combination PKP and phacoemulsification can be considered in patients who are willing to wear contact lenses, desire rapid visual recovery and want to avoid multiple surgeries. Otherwise, sequential surgery after achievement of refractive stability (usually requiring at least one year) is preferred.
If stromal opacification is more anterior, keep in mind that deep anterior lamellar keratoplasty has been shown to produce more predictable refractive outcomes and faster visual recovery if combined with cataract surgery, compared to a combined PKP.15 For patients with severe central corneal scarring—specifically in SJS and graft vs. host—consider extracapsular cataract extraction. If you pursue phacoemulsification, low aspiration settings are advisable. Suturing the wounds in these patients is also important.
No Magic Bullet
As you can see, cataract surgery through a “cloudy” cornea presents many challenges that require careful preoperative planning and intraoperative strategies to achieve a satisfactory visual outcome.
Although no single approach can guarantee success, by using the tips in this article, especially in appropriate combinations, you can minimize many avoidable, disappointing outcomes. REVIEW
Dr. Al-Mohtaseb is an associate professor of ophthalmology and associate residency program director, Cornea, Cataract & Refractive Surgery, at the Baylor College of Medicine in Houston.
Dr. Naguib is a 2nd-year ophthalmology resident at the Baylor College of Medicine.
Neither Dr. Al-Mohtaseb nor Dr. Naguid report any relevant financial interest in any products mentioned.
1. Jeng BH, Dupps WJ, Meisler DM, Schoenfield L. Epithelial Debridement for the Treatment of Epithelial Basement Membrane Abnormalities Coincident With Endothelial Disorders. Cornea 2008;27:10:1207-1211.
2. Narang P, Mohamed A, Mittal V, Sangwan VS. Cataract surgery in chronic Stevens–Johnson syndrome: Aspects and outcomes. Br J Ophthalmol 2016;100:11:1542-1546.
3. He Y, de Melo Franco R, Kron-Gray MM, et al. Outcomes of cataract surgery in eyes with previous herpes zoster ophthalmicus. J Cataract Refract Surg 2015;41:4:771-777.
4. Sykakis E, Karim R, Parmar DN. Management of patients with herpes simplex virus eye disease having cataract surgery in the United Kingdom. J Cataract Refract Surg 2013;39:8:1254-1259
5. Panda A, Krishna SN, Dada T. Outcome of phacoemulsification in eyes with cataract and corneal opacity partially obscuring the pupillary area. Nepal J Ophthalmol 2012;4:2:217-223.
6. Yokokura S, Hariya T, Uematsu M, et al. Efficacy of chandelier illumination for combined cataract operation and penetrating keratoplasty. Cornea 2015;34:3:275-278.
7. Oshima Y, Shima C, Maeda N, Tano Y. Chandelier retroillumination-assisted torsional oscillation for cataract surgery in patients with severe corneal opacity. J Cataract Refract Surg 2007;33:12:2018-2022.
8. Shimomura Y, Hosotani H, Kiritoshi A, Watanabe H, Tano Y. Core vitrectomy preceding triple corneal procedure in patients at high risk for increased posterior chamber pressure. Jpn J Ophthalmol 41:4:251-254
9. Terry MA, Shamie N, Chen ES, et al. Endothelial Keratoplasty for Fuchs’ Dystrophy with Cataract. Ophthalmology 2009;116:4:631-639.
10. Price FW, Price MO. Combined cataract/DSEK/DMEK: changing expectations. Asia-Pacific J Ophthalmol (Phila). 2017;6:4:388-392.
11. Chaurasia S, Price FW Jr, Gunderson L, et al. Descemet’s membrane endothelial keratoplasty: Clinical results of single versus triple procedures (combined with cataract surgery). Ophthalmology. 2014;121:454-458.
12. Price MO, Price DA, Fairchild KM, et al. Rate and risk factors for cataract formation and extraction after Descemet stripping endothelial keratoplasty. Br J Ophthalmol. 2010;94:1468-1471
13. Schoenberg ED, Price FW, Miller J, McKee Y, Price MO. Refractive outcomes of Descemet’s’s’s membrane endothelial keratoplasty triple procedures (combined with cataract surgery). J Cataract Refract Surg. 2015;41:6:1182-1189.
14. Crawford GJ, Stulting RD, Waring GO, Van Meter WS, Wilson LA. The triple procedure. Analysis of outcome, refraction, and intraocular lens power calculation. Ophthalmology 1986;93:6:817-824.
15. Den S, Shimmura S, Shimazaki J. Cataract surgery after deep anterior lamellar keratoplasty and penetrating keratoplasty in age- and disease-matched eyes. J Cataract Refract Surg 2018;44:4:496-503.