As every ophthalmologist knows, elevated intraocular pressure is a risk factor for glaucoma progression. Unfortunately, many retina-related procedures, including anti-VEGF injections and some surgical interventions, can cause an increase in IOP.
In most cases, the increase is temporary, so although the pressure can become quite high, consequences are usually minimal or nonexistent. However, susceptibility to damage in some patients can cause even a brief rise in pressure to be consequential; and in other patients, the brief pressure spike can be followed by a long-term IOP increase. Thus, to avoid glaucoma-related complications, it behooves us to identify patients who may be more susceptible to damage in this situation.
As a retina specialist, I know that my glaucoma colleagues are aware of this issue, and there’s usually good communication between the retina and glaucoma teams. However, sometimes a patient is sent to a glaucoma specialist only after the pressure has been elevated for a while. For that reason, spreading awareness of this issue can be helpful.
Here, I’d like to share some of what we know about this concern, the patients who may be at risk, and what we can do to prevent negative consequences from arising—and treat them if they do. In particular, I’ll discuss anti-VEGF injections, vitrectomy surgery with or without tamponade, the use of a scleral buckle and the use of panretinal photocoagulation laser. (I won’t discuss intravitreal or periocular steroid injections, because those are well known to cause an increase in IOP, and they’ve been extensively documented and discussed elsewhere.)
Anti-VEGF Injections
The idea of anti-VEGF medications comes from the oncology world. The premise was that tumors required a prominent blood supply to continue growing, and it was determined that this angiogenesis was a VEGF-mediated process. Retina specialists rapidly made the association that certain intraocular conditions like age-related macular degeneration were also VEGF-mediated processes. This led to the clinical trials studying ranibizumab, the off-label use of bevacizumab and ultimately the development of aflibercept.
Today, intravitreal injections of anti-VEGF drugs are increasingly common. The IRIS Registry shows that 524,485 patients received 2,419,931 injections in 2016 alone. However, that increasing number of injections has drawn attention to their potential drawbacks—including the potential IOP-related consequences of injecting a volume of material into the eye.
From the beginning, everyone understood that pushing fluid into the eye can cause the pressure to increase in the short term. So, when we first started doing injections, doctors wouldn’t let the patient leave the office immediately afterwards; they’d repeatedly check the pressure for 30 or 40 minutes to make sure the pressure had come back down to a reasonable range before allowing the patient to go home.
For a long time it was thought that those short-term post-injection pressure spikes were the only issue.1-3 (For example, in one study IOP increased during the first hour post-injection and then dropped to only 2 to 3 mmHg higher than the baseline level within 60 minutes in most patients.3) But over time, subset analyses of the data from several clinical trials made it apparent that some of these patients did have long-term increases in IOP—presumably triggered by the injections.4-6
These factors raised a number of questions: Are glaucoma patients more likely to be affected long-term? Does the injection duration and/or frequency matter? Does anti-VEGF medication choice matter? Does the patient’s underlying diagnosis matter? Does injection technique matter? And do preventive techniques work?
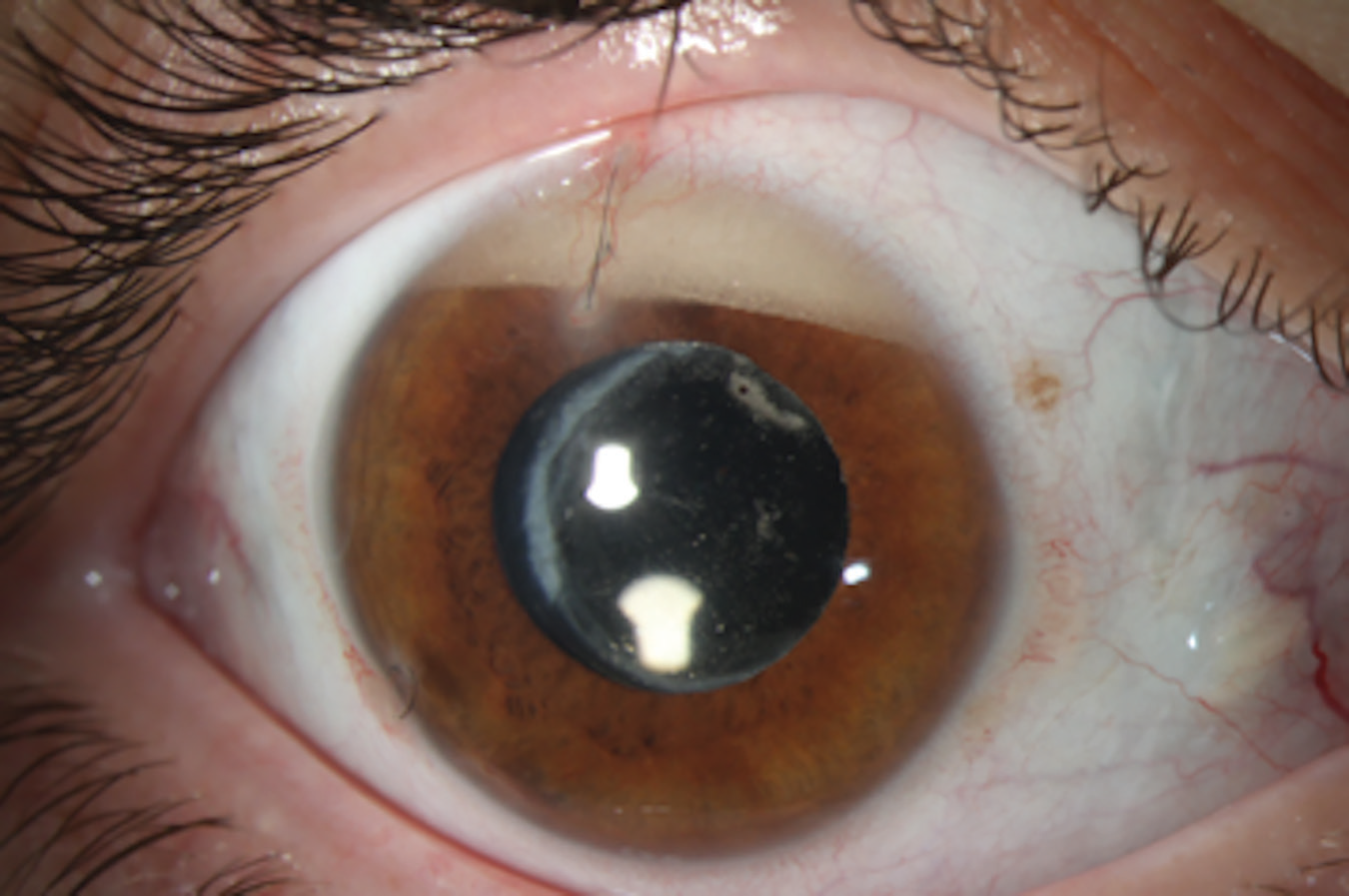 |
|
Emulsified silicone oil that has migrated to the anterior chamber, resulting in a secondary increase in intraocular pressure. |
Who’s at Risk?
At-risk populations were assessed in a meta-analysis of five relevant, randomized controlled trials.7 (Related trials that weren’t included in this meta-analysis were not randomized and had enormous variation in the medications used, the number of injections given, and how they defined “increased pressure,” making them difficult to analyze.) All of the randomized controlled trials included had at least three months of follow-up.
The meta-analysis revealed:
- About 5 percent of the injected patients seemed to be at risk for a sustained increase in IOP.
- The specific anti-VEGF medication used didn’t seem to make a difference.
- A patient’s susceptibility didn’t change no matter how long the patient was followed.
- The underlying indication for the injection didn’t seem to make a difference in the amount of pressure increase.
- Patients with a history of glaucoma were more likely to have a long-term pressure rise than their peers. That’s not a surprise, because if patients already have glaucomatous damage they’re more likely to be affected by a spike in IOP, such as the acute rise in pressure that follows an injection.
More recently, researchers used the IRIS Registry database to look at data from 23,776 patients who got injections either for macular degeneration or for macular edema associated with vein occlusion or diabetes, in 2013, 2014 and 2015.8 This was a well-designed study that involved patients who got injections in one eye but not the other, making it possible to use the other eye as a control. The study authors included patients who received multiple injections of bevacizumab, ranibizumab or aflibercept; they assessed the change in IOP at the one-year mark to see how many of those patients had a significant increase in IOP. (A significant increase was defined as a rise of 6 mmHg over baseline to a pressure above 21 mmHg.)
Their findings included:
- For all groups there was a mean decrease in IOP of almost 1 mmHg in the treated eye and 0.2 mmHg in the fellow (untreated) eye.
- At one year about 2.6 percent of patients had a clinically significant increase in pressure in the treated eye. (About 1.5 percent of patients had a clinically significant pressure increase in the control eye as well.)
- There was a statistically significant difference in pressure increase associated with ranibizumab and bevacizumab compared to aflibercept (a 2.8-percent increase for the first two drugs, but a 1.9-percent increase for the latter). However, it’s hard to know why this difference occurred or what the clinical significance might be.
Our group has also investigated some other factors that could, in theory, impact whether IOP increases following an injection. One such factor is the way in which the surgeon deals with the possibility of reflux of fluid from the injection site. Basically, when you give an injection into the eye, there tends to be some reflux at the injection site because the pressure gets so high. (One study found that, following an intravitreal injection, there was an almost 20 mmHg difference in IOP between eyes that experienced reflux and those that didn’t.9)
Since then, we’ve given more weight to the fact that reflux is a way for the pressure to more rapidly equilibrate. Patients who have no reflux—or in whom reflux is prevented—have a much greater increase in IOP immediately afterwards.
Mitigating the Pressure Increase
Of course, there are numerous ways to manage an increase in intraocular pressure, some of which can be useful in this situation. For example, we know that giving topical anti-hypertensive drops 20 or 30 minutes before the injection reduces the amount of pressure increase following an injection. (See graph, below.)
Another management option is to perform an anterior chamber paracentesis and withdraw some aqueous fluid from the anterior chamber either prior to or immediately following the injection; this blunts the rise in IOP that you get from the intravitreal injection. However, it’s important to know that anterior chamber taps have the potential to lead to complications. There have been occasional reports of unintended consequences such as hitting the lens when creating an anterior chamber paracentesis, so it’s important to consider the risk/benefit ratio before employing this strategy.
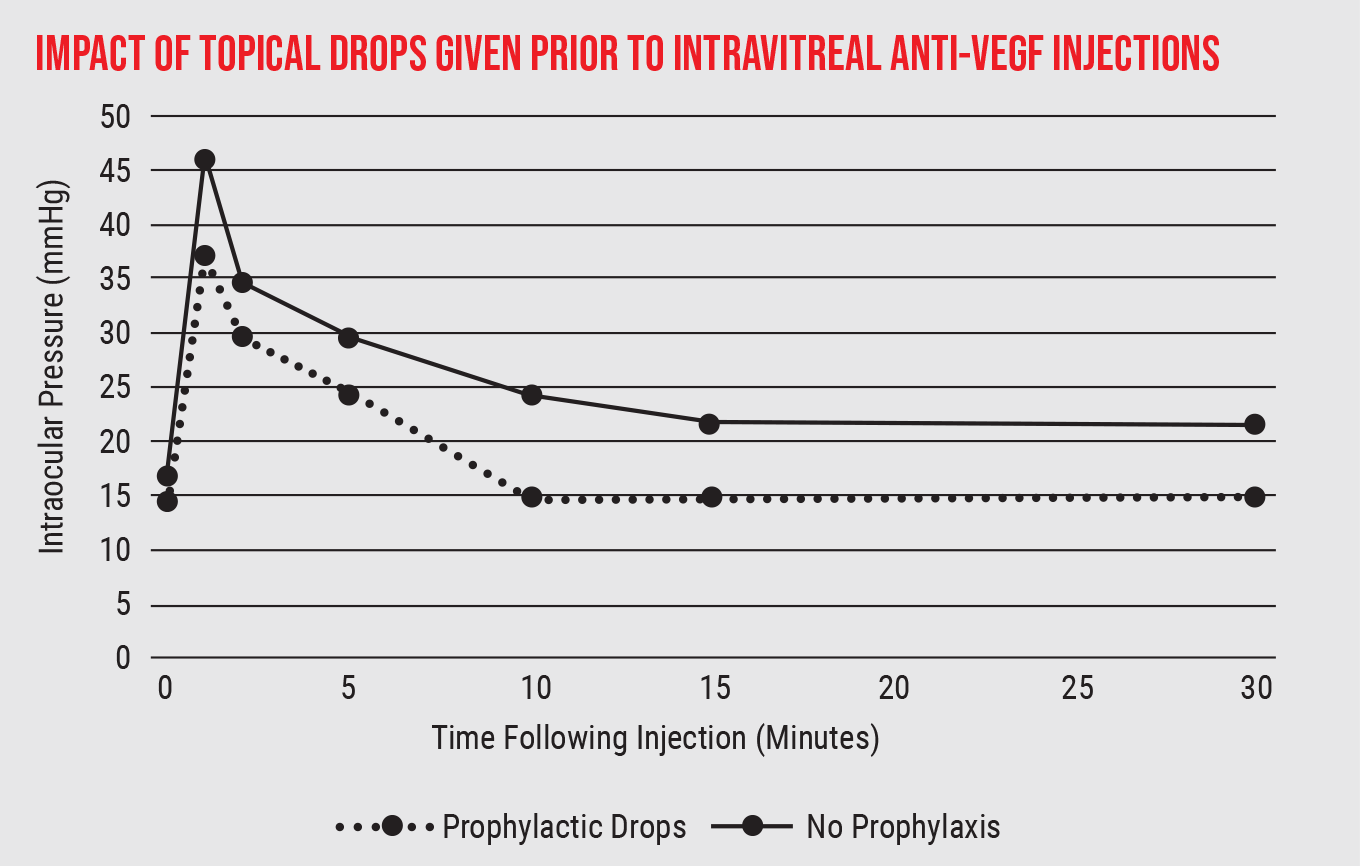 |
|
One way to limit the pressure rise following anti-VEGF injections is to give prophylactic topical drops before the injection. (Data drawn from a review conducted by Peter Bracha, MD, et al, 2018.9) |
In general, I wouldn’t consider creating a paracentesis routinely; the rate of developing a sustained increase in pressure following an injection is so low that this probably isn’t warranted. On the other hand, there are select patients for whom using this approach routinely might be beneficial. If you have a patient with a history of glaucoma, and either the pressure has started to go up or the glaucoma specialist is starting to see changes in the visual field, it might be worth performing an anterior chamber tap to mitigate any pressure increase. In these individuals, having the pressure go from the 10- to 15-mmHg range up to 30 or 40 mmHg—even just for 15 or 20 minutes—could cause some lasting damage to the nerve. I have five or six patients like this for whom I routinely perform an anterior chamber tap before an injection, because they’ve had long-lasting increases in pressure previously.
To summarize what we know about the connection between anti-VEGF injection and long-term IOP increases:
- Glaucoma patients are most likely to be at risk.
- The underlying reason a given patient needs anti-VEGF injections doesn’t seem to make a difference.
- The way the injection is performed can make a difference.
- It’s not yet clear whether the choice of anti-VEGF drug or the frequency of injections makes a difference.
- Steps taken to lower pressure before or after the injection are effective.
Retinal Surgeries: Vitrectomy
Several retinal surgeries are sometimes followed by increased IOP. In particular, this is sometimes seen following vitrectomy, with or without a tamponade; following the placing of a scleral buckle; and after panretinal laser photocoagulation. Let’s look more closely at each of these.
A 2019 population-based study looked at the likelihood of glaucoma developing in age-matched patients who underwent scleral buckle, scleral buckle with vitrectomy, or vitrectomy only.10 The data showed that about 9 percent of patients undergoing a vitrectomy, with or without a scleral buckle, ultimately developed some level of glaucoma. (For comparison, only 1 percent of the general population who didn’t undergo any of these surgical interventions developed glaucoma.) The study concluded that vitrectomy can be associated with increased IOP.
There’s reason to believe that using a gas or oil injection (i.e., a tamponade) to help keep the retina flat postoperatively may contribute to the rise in pressure. (More about the reasons for that shortly.) However, in this study, many of the patients had epiretinal membranes, meaning they would definitely not have received a tamponade. Those patients still showed an increased rate of developing glaucoma after the surgery.
What might explain this? Sometimes anterior vitreous is left in the eye, especially in phakic patients. That can allow aqueous misdirection to occur, where the ciliary body can become rotated; the pressure can go up significantly, the anterior chamber can shallow and uveal effusions can happen. Sometimes this can lead to extensive damage.
A chronic increase in IOP following vitrectomy is probably related to inflammation. Significant anterior chamber inflammation may occur in diabetic patients, or when the surgery was lengthy; that inflammation can result in blockage of the trabecular meshwork. Normally, once the inflammation calms down, the pressure drops. However, if the inflammation continues for a while, posterior synechiae can develop, causing pupillary block. That can prevent aqueous from leaving the eye, resulting in a pressure increase.
Furthermore, in some of the conditions that lead to the need for vitrectomy in the first place, such as diabetic retinopathy or vein occlusions that have led to neovascularization, inflammation from the surgery can cause the neovascularization to worsen. That can block the angle and cause the pressure to increase. Finally, some level of oxidative stress happens during the surgery. That can cause some inflammation, or even direct damage to the trabecular meshwork, which will increase the resistance and reduce the outflow.
Tamponades
Tamponades, often used after vitrectomy to hold retinal tissue in place while it heals, can also cause elevated IOP in some situations. Sometimes the tamponade is accomplished with a gas bubble inside the eye, sometimes with silicone oil. Each option has its own caveats.
The two main types of gas that are used—sulfur hexaflouride and perflouropropane—are both expansile gases that in certain concentrations gradually increase in volume over time. (The former doubles in volume by 24 hours at 25-percent concentration; the latter quadruples in volume by 48 hours at 18-percent concentration.) This can be advantageous, because gas gradually dissipates from the eye; if the gas that remains is gradually expanding, the support it provides will continue for a longer time.
However, this characteristic can backfire. If you put too much gas into the eye, the tendency to expand can cause an increase in IOP. Or, if the concentration you use is incorrect, that can affect the volume doubling or quadrupling time and cause the pressure to increase. (A similar problem can occur if the patient travels via airplane; the change in altitude during flight changes the expansile properties of the gas, increasing the likelihood that the gas will expand more than we want it to. That’s why we always tell patients that they can’t fly with a gas bubble in the eye.)
The benefit of using a gas tamponade is that it dissolves on its own, so there’s no need for additional surgery to remove it. However, there are some situations—such as when there’s a significant amount of scarring or a lot of damage to the retina—in which you need a longer-lasting tamponade. In that situation, silicone oil may be the best choice. It’s beneficial in the sense that it will stay in the eye until you do another surgery to remove it, and it will keep the retina attached while it’s in there. However, there are some potential toxic effects. For example, if the oil flows in front of the iris it can block and eventually close the trabecular meshwork.
There’s also the issue that the oil can sometimes break down or emulsify if it remains in the eye for an extended period. In that situation, some of it may seep forward and block the trabecular meshwork, eventually causing trabecular meshwork scarring and long-term damage. (See first image.) That’s another reason we try to avoid using silicone oil if we can, reserving it for advanced cases with significant disease.
If the oil does end up interfering with outflow and the IOP increases, there are several possible ways to proceed:
• Topical anti-hypertensives are often helpful. In one series of 450 eyes with long-term silicone oil endotamponade, 11 percent developed increased IOP; most (78 percent) were treated only with glaucoma medications.11
• Create an inferior peripheral iridotomy. If the patient in this situation is pseudophakic, this is another approach that may help. (Usually a PI isn’t necessary in a phakic eye because a natural lens is larger than a pseudophakic lens; it tends to do a good job of keeping the oil in the back of the eye.) The PI allows aqueous to come from behind the iris to in front of the iris and the pupil, where it helps to push back and block the oil from coming forward. It’s a little counterintuitive.
Because oil floats, it would tend to rise in the anterior chamber and block a superior PI. So, the PI would be placed inferiorly in one of these patients.
• Remove or replace the oil. If the oil in an eye is starting to emulsify, and the retina is now stable, then you can perform surgery to remove the oil. However, if the retina has appreciable scar tissue, or it appears that it may re-detach, then you may need to do an oil exchange. (In this situation you may need to do this periodically as the oil breaks down.)
• Place a tube. Sometimes this works to relieve the elevated pressure. However, although a tube would normally be placed superiorly, in this situation an inferior quadrant is recommended, for the same reason we’d place a PI inferiorly.12 Oil rises, so if you place the tube superiorly, the oil can go into the tube and either migrate out of the eye through the tube or block the tube completely.
In addition to inferior quadrant placement of the implant, viscoelastic during surgery can prevent intraoperative loss of oil while the patient is in the supine position. Patients may need longer-duration treatment with steroids during the postoperative period.12
• Perform cyclophotocoagulation. If you can’t remove the oil, this can help by partially shutting down the ciliary body so it doesn’t produce as much aqueous. However, this option is pro-inflammatory, so it can create issues of its own.
Scleral Buckle and Laser
Another retinal treatment that can result in an elevated IOP is the placement of a scleral buckle, in which we place a band 360 degrees around the eye. Usually, the squeezing of the eye elevates the pressure, but only in the short term. That can cause the anterior chamber to shallow, but it’s rare that the shallowing is so significant that it causes angle closure. However, sometimes the buckle can be so tight that it affects the blood flow to the eye; that ischemia can lead to inflammation and choroidal effusions or neovascularization, either of which can increase the IOP.13
Usually, corticosteroids, topical anti-hypertensives and cycloplegia are sufficient to address the IOP increase caused by a scleral buckle. Laser iridoplasty may be needed in rare cases. It’s rare that the encircling band needs to be loosened or removed because of the pressure increase.
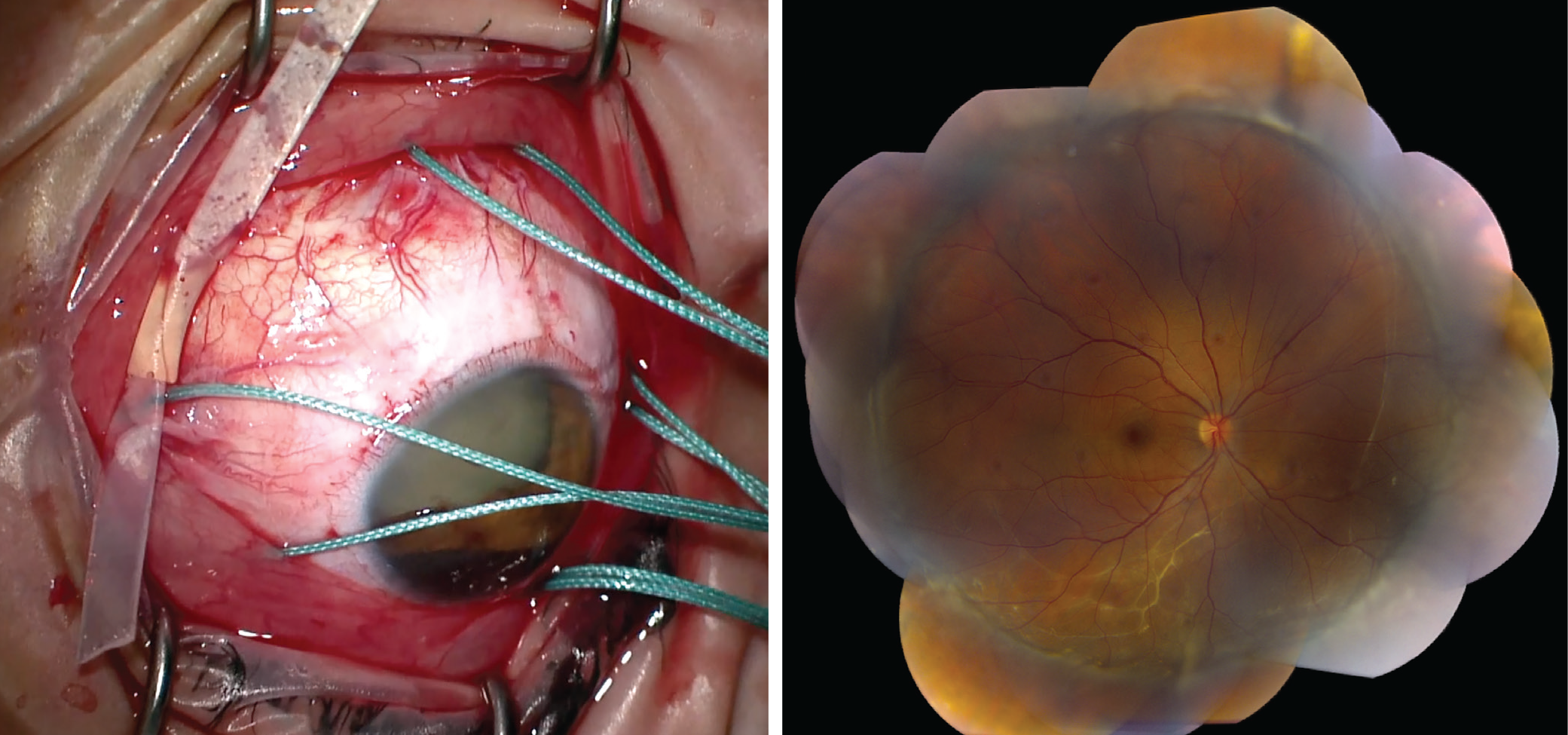 |
|
Left: An intraoperative image of a 360-degree-encircling scleral buckle with sleeve in place, before tightening the band. Right: A montage color fundus photo of a patient with a history of a chronic inferior retinal detachment and subretinal fibrotic bands, demonstrating retinal reattachment after scleral buckle placement. |
The final treatment on the list is the use of panretinal photocoagulation for proliferative diabetic retinopathy. In that case, the laser can be pro-inflammatory and cause the choroid to swell a little bit, pushing everything forward. If the lens-iris diaphragm is pushed forward, that can lead to angle closure. Furthermore, it’s possible for the laser to damage the short ciliary nerves, causing decreased ciliary muscle tone, as well as the release of prostaglandins.
Usually, these effects are transient because the amount of laser and inflammation is fairly limited. As a result, you can often achieve prophylaxis by using a dilating drop and steroid drops.
Working Wisely
As our treatments evolve and proliferate, we must always be on the lookout for unintended consequences of their use—including elevations in IOP. By remaining aware, being careful and maintaining good communication between specialties, we can help ensure that all our patients get the best outcomes possible.
Dr. Williams is part of the vitreoretinal service at Cincinnati Eye Institute. He is the Mary Knight Asbury Chair of Ocular Oncology and an assistant professor in the Department of Ophthalmology at the University of Cincinnati College of Medicine. Dr. Williams is a consultant to Genentech, Castle Bio-sciences and Allergan.
1. Kim YJ, Sung KR, Lee KS, et al. Long-term effects of multiple intravitreal antivascular endothelial growth factor injections on intraocular pressure. Am J Ophthalmol 2014;157:1266–71.e1.
2. Abedi G, Adelman RA, Salim S. Incidence and management of elevated intraocular pressure with antivascular endothelial growth factor agents. Semin Ophthalmol 2013;28:126–30.
3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. New England J Med. 2006;355:1419–31.
4. Bakri SJ, Moshfeghi DM, Francom S, et al. Intraocular pressure in eyes receiving monthly ranibizumab in two pivotal age-related macular degeneration clinical trials. Ophthalmology 2014;121:1102–8.
5. Bressler SB, Almukhtar T, Bhorade A, et al. Repeated intravitreous ranibizumab injections for diabetic macular edema and the risk of sustained elevation of intraocular pressure or the need for ocular hypotensive treatment. JAMA Ophthalmol 2015;133:589-597.
6. Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age-related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122:1802-10.
7. Sniegowski M, Mandava N, Kahook MY. Sustained intraocular pressure elevation after intravitreal injection of bevacizumab and ranibizumab associated with trabeculitis. Open Ophthalmol J 2010;22:4:28-9.
8. Atchison EA, Wood KM, Mattox CG, et al. The real-world effect of intravitreous anti-vascular endothelial growth factor drugs on intraocular pressure: An analysis using the IRIS Registry. Ophthalmology 2018;125:5:676-682.
9. Bracha P, Moore NA, Ciulla TA, WuDunn D, Cantor LB. The acute and chronic effects of intravitreal anti-vascular endothelial growth factor injections on intraocular pressure: A review. Surv Ophthalmol 2018;63:3:281-295.
10. Mansukhani SA, Barkmeier AJ, Bakri SJ, et al. The risk of primary open-angle glaucoma following vitreoretinal surgery—a population-based study. Am J Ophthalmol 2019;201:85-86.
11. Al-Jazzaf AM, Netland PA, Charles S. Incidence and management of elevated intraocular pressure after silicone oil Injection. J Glaucoma 2005;14:40-46.
12. Ishida K, Ahmed II, Netland PA. Ahmed Glaucoma Valve surgical outcomes in eyes with and without silicone oil endotamponade. J Glaucoma 2009;18:325-330.
13. Roa TM, Kanner EM, Netland PA. Glaucoma associated with disorders of the retina, vitreous, and choroid. In Albert D, Miller J, Azar D, Young LH (eds) Albert and Jakobiec’s Principles and Practice of Ophthalmology, 4th Ed. New York: Springer Nature, 2022 (doi.org/10.1007/978-3-319-90495-5_158-1).



